History of Mineralogy. First Part
Although it is impossible in a few paragraphs to trace systematically the development of mineralogy, some of the highlights of its development can be singled out. The emergence of mineralogy as a science is relatively recent, but the practice of mineralogical arts is as old as human civilization.
Natural pigments made of red hematite and black manganese oxide were used in cave paintings by early humans, and flint tools were prized possessions during the Stone Age. Tomb paintings in the Nile Valley executed nearly 5000 years ago show busy artificers weighing malachite and precious metals, smelting mineral ores, and making delicate gems of lapis lazuli and emerald. As the Stone Age gave way to the Bronze Age, other minerals were sought from which metals could be extracted.
We are indebted to the Greek philosopher Theophrastus (372-287 B.C.) for the first written work on minerals and to Pliny, who 400 years later recorded the mineralogical thought of his time. During the following 1300 years, the few works that were published on minerals contained much lore and fable with little factual information. If one were to select a single event signaling the emergence of mineralogy as a science, it would be the publication in 1556 of De Re Metallica by the German physician Georgius Agricola.
This work gives a detailed account of the mining practices of the time and includes the first factual account of minerals. The book was translated into English from the Latin in 1912 by the former president of the United States, Herbert Hoover, and his wife, Lou Henry Hoover. An illustration from this book is reproduced in Fig. 1.3.

FIG. 1.3. Prospecting with a forked stick (A) and trenching (B) in the fifteenth century, (From Agricola, De Re Metallica, translated into English. Dover Publications, New York, 1950.)
In 1669 an important contribution was made to crystallography by Nicolas Steno through his study of quartz crystals. (A portrait of Nicolas Steno is reproduced in Fig. 1.4.)

FIG. 1.4. Portrait of Niels Stensen (Latinized to Nicolaus Steno). Steno was born in Copenhagen, Denmark, in 1638 and died in 1686. (From Scherz, G., Steno, Geological Papers. Odense University Press, 1969.)
He noted that despite their differences in origin, size, or habit, the angles between corresponding faces were constant (see Fig. 1.5).
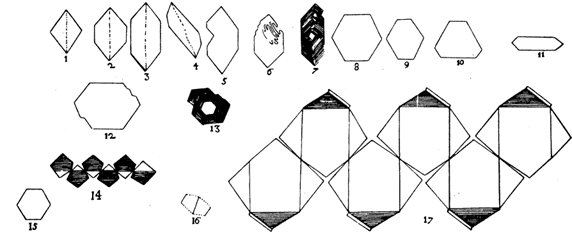
FIG. 1.5. Steno's drawings of various quartz and hematite crystals, illustrating the constancy of angles among crystals of different habits. (From Schafkranovski, J. J., 1971, Die Kristallographischen Entdeckungen N. Stenens, in Steno as Geologist. Odense University Press.)
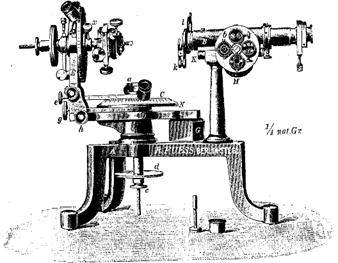
More than a century passed before the next major contributions were made. In 1780 Carangeot invented a device (contact goniometer) for the measurement of interfacial crystal angles (see Fig. 1.7a).
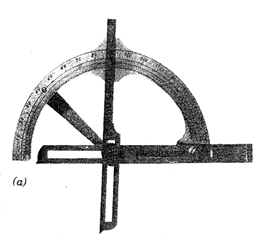
Fig. 17 (a). Examples of instruments used for the measurement of angles between crystal faces. Brass contact goniometer of the Carangeot type. This was used at Harvard University in 1797 (see Frondel, 1983)
In 1783 Rome de I'lsle made angular measurements on crystals confirming Steno's work and formulated the law of the constancy of interfacial angles. The following year, 1784, Rene ). Haüy showed that crystals were built by stacking together tiny identical building blocks, which he called integral molecules (see Fig. 1.6). The concept of integral molecules survives almost in its original sense in the unit cells of modern crystallography. Later (1801) Haüy, through his study of hundreds of crystals, developed the theory of rational indices for crystal faces.
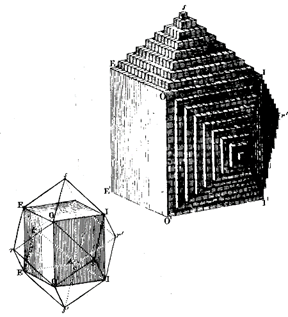
FIG. 1.6. Illustration of the concept developed by R. J. Haüy (1743-1826) of tiny identical building blocks underlying the external form of crystals. In this figure the development of a dodecahedron of garnet is shown. (From Mam, G. M., 1970, Geschichte der Kristallkunde. Reprinted by Sandig, Walluf, Germany.)
In the early nineteenth century rapid advances were made in the field of mineralogy. In 1809 Wollaston invented the reflecting goniometer, which permitted highly accurate and precise measurements of the positions of crystal faces. Where the contact goniometer had provided the necessary data for studies on crystal symmetry, the reflecting goniometer (see Figs. 1.7c and d) would provide extensive, highly accurate measurements on naturally occurring and artificial crystals. These data made crystallography an exact science. Between 1779 and 1848 Berzelius, a Swedish chemist, and his students studied the chemistry of minerals and developed the principles of our present chemical classification of minerals.
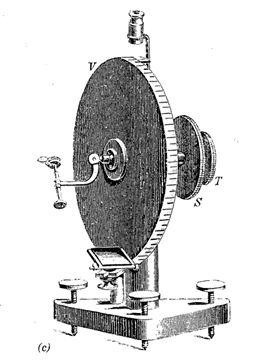
(c) The earliest reflecting one-circle goniometer as invented by W. H. Wollaston in 1809. (From Tschermak, G. and Becke, F., 1921, Lehrbuch der Mineralogie. Hölder-Pichler-Tempsky, Vienna.) (d) A two-circle reflecting goniometer as developed in the latter part of the nineteenth century
In 1815 the French naturalist Cordier, whose legacy to mineral science is honored in the name of the mineral cordierite, turned his microscope on crushed mineral fragments in water. He thereby initiated the immersion method that others, later in the century, developed into an important technique for the study of the optical properties of mineral fragments. The usefulness of the microscope in the study of minerals was greatly enhanced by the invention in 1828 by the Scotsman, William Nicol, of a polarizing device that permitted the systematic study of the behavior of light in crystalline substances. The polarizing microscope became, and still is, a powerful determinative tool in mineralogical studies. An early model is illustrated in Fig. 1.8. In the latter part of the nineteenth century Fedorov, Schoenflies, and Barlow, working independently, almost simultaneously developed theories for the internal symmetry and order within crystals which became the foundations for later work in X-ray crystallography.
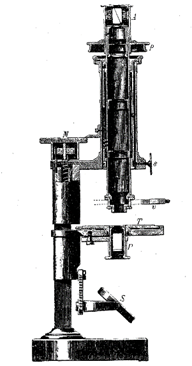
FIG. 1.8. Polarizing microscope as available in the mid-nineteenth century. (From Tschermak, G. and Becke, F., 1921, Lehrbuch der Mineralogie. Hölder-Pichler-Tempsky, Vienna.) Compare with Fig. 7.12
The most far-reaching discovery of the twentieth century must be attributed to Max von Laue of the University of Munich. In 1912 in an experiment performed by Friedrich and Knipping at the suggestion of von Laue, it was demonstrated that crystals could diffract X-rays. Thus was proved for the first time the regular and ordered arrangement of atoms in crystalline material. Almost immediately X-ray diffraction became a powerful method for the study of minerals and all other crystalline substances, and in 1914 the earliest crystal structure determinations were published by W. H. Bragg and W. L. Bragg in England. (Their photographs are given in Fig. 1.9.)


FIG. 1.9. Portraits of (a) Sir William Henry Bragg (1862-1942) and (b) his son Sir William Lawrence Bragg (1890-1971). Father and son received the Nobel Prize for Physics in 1915. Both men are eminently known for their researches in the field of crystal structure by X-ray methods. (a from Godfrey Argent, London, photograph by Walter Stoneman; b from Times Newspapers, Ltd., London.)
Modern X- ray diffraction equipment with online, dedicated computers has made possible the relatively rapid determination of highly complex crystal structures. The advent of the electron microprobe in the early 1960s, for the study of the chemistry of minerals on a microscale, has provided yet another powerful tool that is now routinely used for the study of the chemistry of minerals, synthetic compounds, and glasses. Electron microprobes (see Figs. 1.10 and 1.11) can provide accurate, many-element analyses of solid materials in a grain size as small as about one micrometer (0.001 mm).
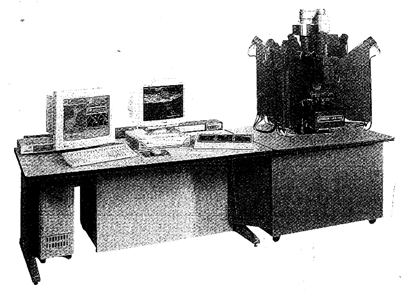
FIG. 1.10. A computer-automated electron microprobe manufactured by Cameca, Courbevoie, France. The electron beam column and X-ray spectrometers are on the right with computer control and readout systems on the left. This is a Cameca SX 100 Electron Probe Microanalyzer. (Courtesy of Cameca Instruments, Inc., Trumbull, Ct)
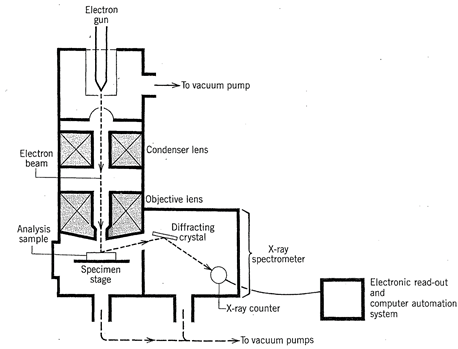
FIG. 1.11. Schematic cross section through the electron optical column and X-ray spectrometer of an electron microprobe
The majority of mineral analyses now produced are made by electron microprobe not only because of the fine focus of the electron beam of the instrument, but because the analyses can be made in situ — on specific mineral grains in polished sections and polished thin sections of rocks. This has eliminated the laborious process of mineral separation and concentration, which is a requirement for several other mineral analysis techniques (see Chapter 7 for further discussion of analytical techniques).
Date added: 2022-12-31; views: 672;
