Plastics, Thermosetting. A Polyimide Molecule
Thermosetting plastics, or thermosets are a type of polymer, usually made by mixing two or more suitable liquids together. Thermosets are usually supplied in the form of partly polymerized precursors or as mixtures of monomer and polymer.
When mixed, the liquids undergo a chemical reaction and form a hard solid. Polymers are made up of long molecules that resemble chains. In thermosetting polymers, the long molecular polymer chains are linked by covalent bonds located in three dimensions by cross-linking. When the liquid precursors are mixed together, bonds start to form between the polymer chains during the curing process.
This process continues until all the chains have joined together forming a single giant molecule. The molecule’s gigantic size makes it solid. Chemicals, applied heat or radiation may be used during manufacture to bring about the polymerization process (curing).
Thermosets have a critical temperature and once heated above this temperature and molded into shape, they will stay in that form. Usually, thermosets cannot be altered by further heating. However, although the two-step curing process forms a three-dimensional structure with crosslinked bonds that do not break down on heating, at very high temperatures thermosets will break down permanently.
The molecular structure of thermosetting polymers determines their properties. The cross-links that exist in their molecular structure stop the molecular chains from sliding past one another, and give thermosets a higher modulus and better creep resistance. Once it has begun, the crosslinking process cannot be reversed. This results in materials that cannot be recycled by remelting. Thermosets are usually more brittle, less flexible and impact-resistant than thermoplastics although they possess better abrasion and dimensional properties.
Thermosets are similar to elastomers—at room temperature, the polymer chains in thermosets are below their glass transition temperature (Tg), making them hard and brittle. However, the polymer chains in elastomers are above their Tg at room temperature, and this factor makes them rubbery. The Tg is the point at which a polymer changes from a rigid solid to a rubber. Some polymers are used above their Tg while others are used below it.
Thermosets in the form of phenolic resins were first developed in the U.S. by the Belgian emigre chemist, Leo Baekeland in 1907 and first patented in 1909. They were developed contemporaneously in Britain by Sir James Swinburne. Baekeland named his invention Bakelite. He reacted phenol and formaldehyde under controlled conditions, producing an amber-colored resin to which he added a filler such as wood flour or cotton flock, producing dark-colored moldings: normally dark brown, dark red or dark green.
Products made from phenolic resins possess good electrical resistance and mechanical properties, and Bakelite was immediately utilized as an insulating material in electrical applications such as plugs and insulators. As phenolics are very difficult to ignite and are thermosets, they were also used for purposes that require materials that need to be able to resist high temperatures, such as in thermos flasks.
Other thermosetting polymers developed since the advent of phenolic resins in the early twentieth century include amino resins (including thiourea- urea-, urea- and melamine-formaldehydes), unsaturated polyesters, polyurethanes, and epoxy resins. Thiourea-urea formaldehydes were discovered in 1924 by Edmund Rossiter, while working for the British Cyanides Company and became popular for a range of decorative tableware called Bandalasta.
By 1929, urea-formaldehyde had been developed with better properties. Baron Justus von Liebig, a German chemist, discovered melamine resin in 1834, but melamine-formaldehyde polymer was not patented until 1935.
The American Cyanamid Company produced it commercially in 1939. As melamine-formaldehyde was water resistant and tougher than urea-formaldehyde and transparent, it became possible to impregnate patterned papers for surfacing decorative laminates such as Formica and Warerite. These led the way to easy-to-care for surfaces, particularly in the kitchen. Polyimides were introduced in the 1960s and cyanate esters are now under development (see Figure 13).
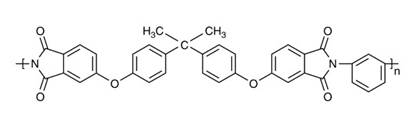
Figure 13. A polyimide molecule
As thermosets are cross-linked polymers that do not melt, they have to be molded under pressure; for example, by compression molding, transfer molding and various injection molding techniques. Thermoset composite materials can be made in a number of ways, for example by using processes such as resin transfer molding, centrifugal molding and autoclave molding.
The manufacture of thermoset composite materials may be rather labor intensive in cases where some components have to be hand laid. For example when making a carbon fiber-thermosetting resin composite, the carbon fiber might be laid by hand, usually in the form of a prewoven fiber. The thermosetting resin, such as epoxy, will then have to be applied and the system allowed to cure. Another type of thermoset composite material such as glass-reinforced fiber (GRP) might use short fibers that are embedded in a polyester or epoxy resin matrix.
The chemistry of processing of thermosets is more complicated than that needed to process thermoplastics, which need only to be melted and cooled. As thermosets are transformed from materials that can be fused and are soluble to highly inflexible cross-linked resins that are impossible to mold, they have to be manufactured during the cross-linking process.
Unsaturated polyester resins have a maximum service temperature of around 100oC and vinyl esters and epoxies a temperature of about 150oC. For this reason, high-performance systems suchpyromelltimide-type polyimides, cyanate esters and bis-maleimides have been developed, as resin matrices that can be used at higher temperatures are needed in certain applications.
These high-performance systems form thermosets that can be used at temperatures of up to 250oC and molded at around 250-300oC. In contrast, high-performance thermoplastics have to be processed at higher temperatures of up to 450oC.
Jon Jakob Berzelius produced the first polyester resin (polyglycerol tartrate) in 1847. A range of polyester resins is now available, including the Marco and Crystic unsaturated polyester resins, which were developed by Scott Bader in 1946. The first commercial use of low-pressure resins to make reinforced polymer composites occurred in 1942 in the form of glass-cloth-reinforced resin radomes made for aircraft in the U.S.
By the late 1940s GRP, more commonly known as fiber glass, was used commercially, with many of the earliest developments derived from making hulls. The first car with a fiber-glass body, the Corvette, was made in 1953. Fiber glass was also used for corrugated roofing, decorative moldings and (unsuccessfully) for window frames and baths. Certain items of furniture, such as stackable chairs, are often made using fiber glass and first appeared in the 1950s.
Epoxy resins are also used with glass fiber to make a composite low-pressure reinforced molding material. Epoxy resins were first developed in the 1930s by Pierre Castan and became commercially viable in 1939 with IG Farben’s patent concerning liquid polyepoxides. The initially high cost of production of epoxy resins as compared with polyesters limited their use until later improvements in production methods. Today they are particularly advantageous for space applications, due to their light weight and excellent electrical properties.
Thermosets are particularly suitable as materials of choice for making big components such as boat hulls, as the liquid precursors from which they are made are not very viscous and so can flow easily, filling up large moulds without needing great injection pressures. Once they have filled up the mould, the precursors then react to form a solid product.
Thermosets such as phenolics are very difficult to recycle because of their high temperature resistance, inability to soften on melting, and their insolubility. However Bakelite (phenol formaldehyde) will biodegrade if broken, provided it has been filled with organic fillers such as woodchip, which can become the site for biodegradation to occur.
Thermoset composites are used in a variety of products from GRP furniture, boat hulls, and sheet-molded compounds (SMC) to carbon fiber-reinforced plastic (CFRP) tennis rackets, Formula One car bodies and aircraft radomes. These are currently difficult to recycle. Furniture and sports equipment may be recycled in the form of repair or reuse, but eventually, once beyond repair, they end up on the scrapheap for the foreseeable future.
Thermoset composite materials can be burned as fuel or to give energy (pyrolysis). However, because many of these fiber-based products such as CFRPs are so expensive to produce, ways have been found to recycle them by grinding and reusing them as fillers, and by selective chemical degradation.
Date added: 2023-11-02; views: 575;
