Plastics, Thermoplastics. Chemical formula
Thermoplastic polymers are perhaps some of the best-known plastics, as they are used for a variety of household items and packaging materials. They comprised 10 percent of the global chemical industry in 1995 (around 90 million tons). Initially made from a process based on coal, they are now usually made from oil-based products.
Thermoplastics include plastics developed as early as 1877 when polymethylmethacrylate (PMMA, or acrylic) was first formed by Rudolph Fittig, developed by Otto Rohm, a German chemist, but not commercially developed by Rohm & Haas until 1928 and later by Rowland Hill and John Crawford at ICI in 1934. In the 1930s other thermoplastics were developed, such as polyethylene by ICI in the U.K., and nylon 6 and nylon 6,6 by Wallace Hume Carothers at DuPont in the U.S. Polyethylene is now commonly used as a packaging material and synonymous with the plastic bag.
Nylon was first used to make toothbrush bristles in 1938, then ladies’ stockings in 1939. Thermoplastic polypropylene, polyvinyl chloride (PVC), and polystyrene are common packaging materials today. Carothers had worked on polyesters and his work was progressed by John R Whinfield and James T Dickson at the Calico Printers Association in the U.K., who produced the polyester fiber Terylene in 1941. This material is widely used to make clothing and soda bottles.
Thermoplastics also include certain resins such as polyether ether ketones (PEEK) which are used to make composite material with superior mechanical properties although at a high cost, therefore they are normally only used in high-technology fields such as aerospace (see Composite Materials).
With thermoplastics, increased strength and thermal resistance comes at an increased price, with polystyrene as the cheapest, least thermal resistant and weakest thermoplastic, followed by low-density polyethylene, high-density polyethylene, polypropylene, polymethyl methacryalate (PMMA, or acrylic), high-impact polystyrene, acrylonitrile butadiene styrene (ABS*) (used for car bumpers), polyesters, polycarbonates and polysulfones to nylon (a polyamide) which has the highest strength and thermal resistance but is also the most expensive. Polycarbonates have high impact strength, hardness, toughness, and resistance to temperatures between about —40°C and 145°C. Polysulfones are heat-resistant at temperatures of up to 150°C
Thermoplastics can be molded to set into a certain shape, but they can then be reshaped after reheating. This is due to their molecular structure, which consists of long chains of molecules held together by weak intermolecular forces. They have a structure almost resembling spaghetti, bound together by the weak Van der Waals forces. Some cross-linking can occur with side groups such as the vinyl group in PVC. The orientation of these side groups will have a great influence on how the polymer will behave and its properties such as strength.
Thermoplastics have to be processed at a higher temperature than thermosetting plastics; this may be more than 400oC for ‘‘high temperature’’ thermoplastics.
The weak intermolecular forces in thermoplastics make them relatively easy to process using a variety of methods ranging from extrusion, vacuum and blow molding to perhaps the most common process, injection molding. They are pliable, and easily shaped and molded. The molding process becomes easier as thermoplastics become hotter, but at some point they will melt.
Thermoplastics have a glass transition temperature (Tg) above room temperature, whereas that of elastomers is below room temperature. The glass transition temperature is where a polymer changes from a rigid solid to a rubber. Below this temperature the polymer becomes hard and brittle, like glass. Some polymers are used above their glass transition temperatures while others such as polystyrene and PMMA are used below.
Thermoplastics can also be blended with other polymers, such as elastomers, to enhance certain properties such as toughness. These are known as copolymers and they can be engineered for specific purposes.
There is also a class of thermoplastic elastomers with physical rather than chemical cross-links. An example of such a material is a thermoplastic elastomer, polyurethane.
Thermoplastics are easy to mold and can be shaped. They are resistant to deformation but will eventually permanently deform or break. They are hard and brittle below their Tg, but pliable and soft above it. Crystalline polymers melt below their glass transition temperature, whereas amorphous polymers are hard and brittle below their Tg, but become flexible and rubbery above it.
Most thermoplastics are a mix of a crystalline and an amorphous structure. Hard plastics such as polystyrene and PVC are used at temperatures below their Tg whereas flexible plastics such as polypropylene are used above it. Some thermoplastics such as polypropylene, nylon, polyketones and syndiotactic polystyrene are highly crystalline, whereas others such as PMMA, polycarbonates and atactic polystyrene are highly amorphous due to their polymer structure and the intermolecular forces.
Thermal or mechanical methods can be used to alter the crystalline structure of a thermoplastic. If a polymer is cooled slowly from its melting point, a higher degree of crystallinity is more probable. However when polymers are cooled quickly from the melt, the amorphous chains may be frozen into the solid. This is because the chains will not have had enough time to untangle from their melted form to separate and form crystals.
Thermoplastic composites are frequently tougher than thermoset composites, but although they do not possess enhanced fatigue or static properties and they may also have worse compression strength, they are more resistant to moisture and a range of industrial solvents than thermosets.
Thermoplastics are made from molecular chains with various types of stereochemical arrangements possible that can confer different properties on the polymer. These are called atactic, isotactic and syndiotactic. In an isotactic polymer, all the substituent groups lie on one side of the molecular chain. In isotactic polypropylene, for example, the methyl (CH3) groups are on the same side of the molecular chain. This arrangement allows the molecular chains to pack together more easily giving a crystalline structure that is strong, stiff and brittle (see Figure 9).
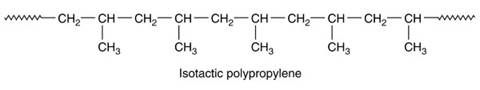
Figure 9. Chemical formula
Atactic thermoplastics have substituent groups that are randomly placed on both sides of the chain for example the methyl groups in atactic polypropylene (see Figure 10). This arrangement means that the chains are unable to pack together, resulting in an amorphous structure that makes the polymer tough and rubbery. This results in a form of toughened polypropylene.
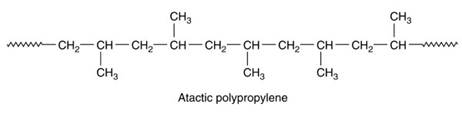
Figure 10. Chemical formula
Syndiotactic polymers have repeating units on either side of the molecular chain backbone, alternating with each other (see Figure 11). In syndiotactic polystyrene, the chains are able to pack tightly together giving a crystalline structure whereas the atactic form of polystyrene is amorphous as the chains cannot pack together so tightly.
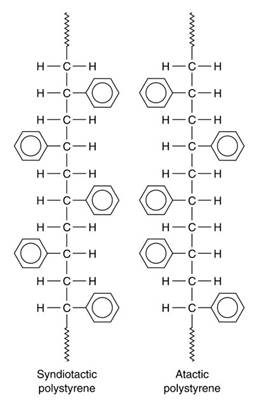
Figure 11. Chemical formula
Thermoplastics can be recycled, but require careful sorting to separate the various different polymers. If different grades are mixed the resultant recycled polymer will have variable properties. Normally, recycling a polymer will involve some deterioration in its properties.
Thermoplastics have flexibility and so are useful where this is needed, for example in squeezable washing-up bottles. The first of these made was the 1958 ‘‘Squeezy’’ bottle, which was made of polyethylene (although with metal ends at this point). Thermoplastics can be blow-molded into a variety of shapes and polyethylene terepthalate (PET) is a popular thermoplastic used to make ‘‘pop’’ bottles.
Thermoplastics are excellent materials for coextrusion—a process widely used in packaging to make multilayered sheet with different properties in the different layers—tough on the outside and impermeable on the inner layers. This technique is popular for producing packaging materials.
Although polyethylene is self-toughening, different formulations of polyethylene have been developed such as low-density polyethylene, the earliest type developed first in 1933, high-density polyethylene, and now ultrahigh molecular weight polyethylene.
Polypropylene is used for packaging and in cars and polypropylene fibers are also used for clothing, carpets and nonwoven fabrics. Impact-resistant copolymers of polypropylene are used to make car bumpers as well as for medical use.
Teflon (polytetrafluoroethylene or PTFE) is used to make products that operate at high temperatures because of its heat-resistant properties. It has excellent chemical, electrical, mechanical and thermal properties and is also chemically inert. Almost nothing sticks to Teflon hence its use for non-stick frying pans. As it is very heat resistant, it is often used in space applications, for example as a material to protect the deployment rods of the solar arrays which replaced the original arrays during the first servicing mission of the Hubble space telescope in 1993.
The future of thermoplastics demands improved recyclability, toughness, repairability and ‘‘smart applications’’ such as self-healing abilities and perhaps inbuilt obsolescence and, ideally, biodegradability. Bioengineered thermoplastics—the class of so-called biopolymers—are an exciting new development. Polyesters are already engineered to make specialist fibers required for sporting applications. More improved engineered fibers are continuously being developed. In the future they will also have to possess an environmentally friendly life-cycle.
Date added: 2023-11-02; views: 683;
