Microstructure of mature bone in both transverse section
Bone is a Bone specialized connective tissue that has a calcified extracellular matrix designed to provide a rigid framework for support and protection of soft tissues of the body. Bones also form a lever system that, when combined with skeletal muscle, makes locomotion possible. In addition to these functions, bone serves as a reservoir for developing blood cells and for ions (i.e., calcium phosphate) that can be stored or released in a regulated manner to maintain homeostasis of body fluids.
Cells embedded within the calcified matrix occupy spaces called lacunae and are called osteocytes, whereas cells on peripheral surfaces of bone are called osteoblasts. Processes of adjacent osteocytes occupy thin channels in the calcified matrix called canaliculi that allow the cells to communicate via gap junctions. Cells involved in the remodeling of calcified bone matrix are called osteoclasts. Osteoclasts are often seen within surface depressions in the matrix known as Howship’s lacunae.
The extracellular matrix of bone consists of both inorganic components—hydroxyapatite crystals and amorphous calcium phosphate—and organic components—including type I collagen, proteoglycans, and glycoproteins (sialoprotein and osteocalcin).
The surfaces of bone are completely covered by layers of osteoprogenitor cells. Periosteum covers the external surface of bone and consists of osteoprogenitor cells and fibroblasts embedded in a dense fibrous connective tissue layer. Bundles of collagen in the periosteum called Sharpey’s fibers penetrate the bone and anchor the periosteum to the bone matrix. The internal surfaces of bone are lined by endosteum, which consists of a single layer of osteoprogenitor cells and delicate connective tissue.
Two types of bone can be distinguished by gross examination: compact bone and cancellous or spongy bone. Compact bone consists of consecutive layers or lamellae that are laid down parallel to one another or of lamellae in concentric circular arrays around a vascular channel. The former constitute inner and outer circumferential lamellae in long bones, whereas the concentric lamellae surrounding blood vessels form a microscopic unit referred to as a Haversian system or osteon. Collagen fibers within individual lamellae are parallel to one another but are laid down perpendicular (or in orthogonal array) to those in adjacent lamellae, thereby giving greater strength to the bone as additional lamellae are formed. Surrounding each osteon is an amorphous layer called cementing substance, which consists mainly of ground substance and a few collagen fibers. Interstitial lamellae are irregularly shaped groups of parallel lamellae that lie between adjacent osteons as a result of incomplete removal of osteons during growth and remodeling of bone.
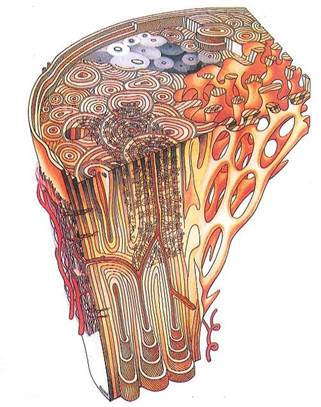
Fig. 5-1. Microstructure of mature bone in both transverse section (top) and longitudinal section and areas of compact and cancellous bone. Central area in transverse section simulates a microradiograph, with densities reflecting variations in mineralization. Note general construction of osteons, distribution of osteocyte lacunae, Haversian canals and their contents, resorption spaces, and different views of structural basis of bone lamellation.
Compact bone forms the surfaces of all bones and therefore surrounds cancellous or spongy bone that is found inside the epiphyses (ends of long bones), or within flat bones. Spongy bone consists of a three-dimensional array of spicules or trabeculae made up of parallel lamellae. In long bones, spongy bone serves to transmit the load of weight bearing from the larger articular surface to the narrower diaphyseal shaft.
During the formation of each bone, two types of bone tissue appear. The first is called primary, woven, or immature bone. It is identified by an irregular orientation of collagen fibers, a low mineral content, and a high proportion of osteocytes. Immature bone tissue is temporary and is replaced, in the adult, by secondary bone tissue, which shows a characteristic lamellar arrangement, a higher mineral content, and a lower proportion of osteocytes.
The formation of bone tissue occurs by either intramembranous or endochondral ossification. Intramembranous bone formation takes place within richly vascularized condensations of mesenchymal tissue, where mesenchymal cells differentiate into osteoblasts and produce primary or immature bone matrix. This deposition of immature bone is followed by remodeling with the formation of secondary or mature bone. In endochondral bone formation, condensations of mesenchymal cells first give rise to a hyaline cartilage modelof the bone. Calcification of the peripheral diaphyseal region in the cartilagenous model leads to formation of a periosteal collar followed by degeneration of internal chondrocyt.es.
An osteogenic bud containing blood vessels penetrates the periosteal collar and brings osteoblasts into the degenerating cartilagenous matrix, thereby forming the primary ossification center. Later in embryonic development, secondary ossification centers develop at the epiphyseal ends of eachlong bone. As growth of the long bone continues, the remaining hyaline cartilage becomes restricted either to the articular surfaces (which persist throughout adult life) or to the epiphyseal plate, which serves as a region permitting growth in bone length until adulthood. At this time a boney union of the diaphysis and epiphyses occurs, and no further increase in growth of the long bone can take place.
A drawing of osteons illustrating organization of mature and developing Haversian systems
Fig. 5-2. Macrophotograph of head of humerus demonstrating spongy (cancellous) bone in marrow cavity and compact bone externally.
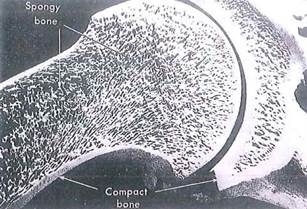

Fig. 5-3. SEM of long bone showing endosteal surface adjoining marrow cavity and demonstrating junction between cancellous bone with trabeculae (upper left) and adjacent compact bone (lower right). (X 30.).
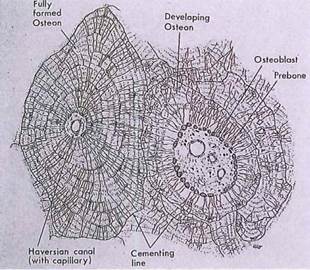
Fig. 5-4. A drawing of osteons illustrating organization of mature and developing Haversian systems. Observe arrangement of lamellae and radial distribution of canaliculi between lacunae in adjacent lamellae.
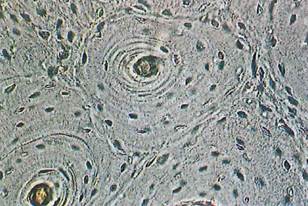
Fig. 5-5. LM of ground bone showing osteon in cross section. Compare it to Fig. 5-4, then locate lacunae of osteocytes, Haversian canal, lamellae, and cementing line surrounding osteon. (x 150.).
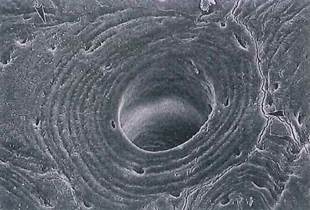
Fig. 5-6. SEM of osteon in cross section. Lamellae, lacunae, and cementing line (arrowheads) of the osteon are clearly seen. (X450.).
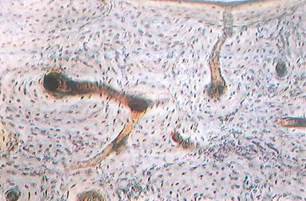
Fig. 5-7. LM of ground bone (from diaphysis of long bone) seen in cross section. Marrow cavity bordered by inner circumferential lamellae is seen at top. Identify Haversian canals, osteons, Volkmann’s canals, and osteocytes in lacunae. (x 100.).
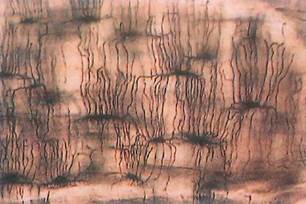
Fig. 5-8. LM section of ground bone. Observe that lacunae (containing osteocytes) are joined by canaliculi through which osteocytes may maintain physiologic and anatomic contact with each other. (X650.).

Fig. 5-9. High-magnification LM of Haversian canal in longitudinal section (top) of ground bone revealing openings of cana- liculi (arrowhead). Osteocytes in lacunae (below) receive metabolites from vessels in Haversian canal by way of diffusion and intercellular coupling (gap junctions) of osteocytic processes within canaliculi. (x 650.).
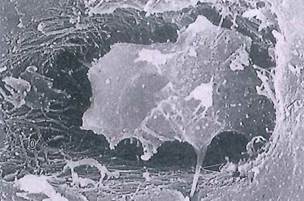
Fig. 5-10. Osteocyte within lacuna (SEM) extending a cytoplasmic process (arrowhead) into canaliculus below. Observe unmineralized collagen, called osteoid (o), surrounding cell body. (SEM; X 9,000.).
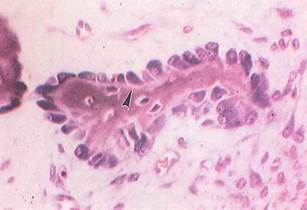
Fig. 5-11. Columnarlike osteoblasts in pig embryo arrayed on bone spicule during intramembranous bone formation (LM). Osteoblasts are very basophilic because of production and secretion of protein (collagen, etc,). Osteoid (arrowhead) appears as lightly stained areas beneath osteoblasts. (H&E; X400.).
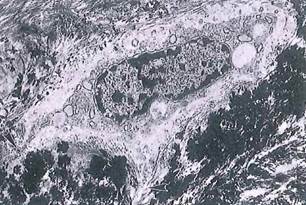
Fig. 5-12. Bone formation by osteoblasts seen by ТЕМ. Unmineralized periosteum is at upper left. Electron-dense bone matrix (mineralized) surrounds cytoplasmic processes (arrowheads) extending into canaliculi. (x 6,300.).
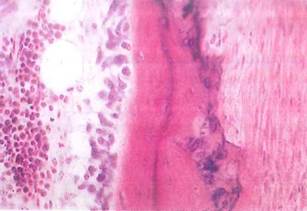
Fig. 5-13. LM showing compact bone (center) with osteocytes in lacunae. To left of bone are osteoblasts, elongate basophilic cells involved in secretion of bone matrix. Periosteum covers bone (on right). (H&E; x250.).
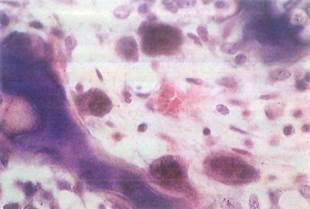
Fig. 5-14. Multinucleated, eosinophilic osteoclasts seen by LM. One osteoclast (lower center) is in a Howship's lacuna, a cavity or depression in bone caused by cell's action in resorbing bone matrix. (H&E; X400.).
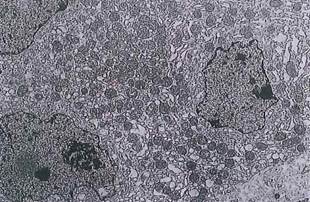
Fig. 5-15. Osteoclast as seen by ТЕМ. Notice numerous mitochondria and multiple nuclei. Bone matrix is at upper left, (x 4,200.).

Fig. 5-16. ТЕМ of part of an osteoclast in contact with bone (left), which it is resorbing. Folds of osteoclast plasmalemma, called "ruffled border," serve to increase surface area of cell, which is active in resorption. Note collagen fibers (arrowhead) in bone matrix. (x 8,300.).
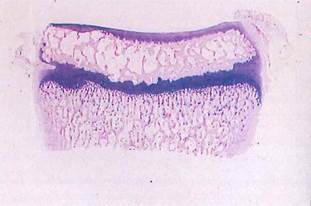
Fig. 5-17. Macrophotograph of long bone. Thin articular cartilage covering epiphysis is seen above, while deeply basophilic cartilage of epiphyseal plate separates epiphysis from metaphysis. (H&E; X4.).
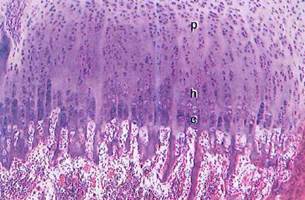
Fig. 5-18. LM of epiphyseal plate. Zone of resting cartilage (r) is seen at top and marrow cavity at bottom. Spicules of calcified cartilage matrix with thin depositions of newly formed bone on their surfaces project into marrow cavity, p, Zones of proliferation; h, hypertrophy; c, calcification. (H&E; x 100.).
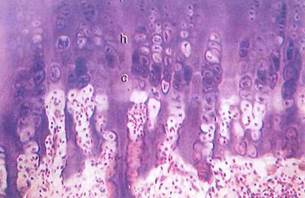
Fig. 5-19. LM of epiphyseal plate showing ("top to bottom) zone of proliferation (p), where chondrocytes in lacunae divide, producing growth of cartilage; zone of hypertrophy (h), where cells accumulate glycogen; and zone of calcification (c), where chondrocytes die and matrix calcifies. Newly formed (eosinophilic) bone is seen on some spicules of calcified cartilage. (H&E; X 200.).
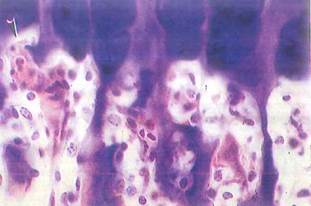
Fig. 5-20. Higher magnification LM of epiphyseal plate. Hypertrophied chondrocytes have died in zone of calcification, and invading vascular buds (arrowhead) from marrow have opened lacunae in calcified cartilage. Bone is deposited by osteoblasts on remaining spicules of cartilage. Remodeling of bone / cartilage matrix by osteoclasts is an active process in this region. (H&E; X 300.).
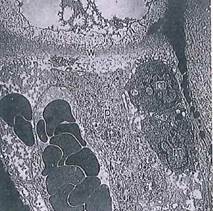
Fig. 5-24. ТЕМ of zone of calcification of epiphyseal plate. When chondrocytes die as a result of changes in matrix, osteoclasts and capillaries in the vicinity break down noncalcified collagenous crosswalls between lacunae. Osteogenic cells then migrate into resulting cavities in calcified cartilage and begin deposition of new bone, w, Lacuna crosswall decalcified; p, osteoprogenitor cell; m, monocytes, (x 1,750.).
Date added: 2022-12-11; views: 884;
