Light Triggers Plant Adaptation and Acclimation to the Environment
Photoreceptors. Because of its role as an energy source, light is arguably the most important environmental factor for a plant. Consequently, perception of light and translation of the respective signals control many aspects of plant development and are crucial for adaptation to diverse habitats. The sensing of light conditions is enabled by a range of receptors, which are specific for particular wavelengths of the visible spectrum (Fig. 3.18).
Phytochromes are receptors for red and far-red light, cryptochromes and phototropins are receptors for blue light. The photoreceptors for red and blue light principally consist of a protein and a chromophore. The chromophores are molecules absorbing light of the respective wavelengths (for example, flavin adenine dinucleotide (FAD) and tetrapyrroles). During evolution they have been recruited for a wide range of biological functions. Upon photon absorption the chromophores undergo conformational changes that are transmitted to the protein and thereby change its activity. For UV light a special photoreceptor has recently been identified (UVR8) which, because of the UV absorption by aromatic amino acid residues, does not require a chromophore.
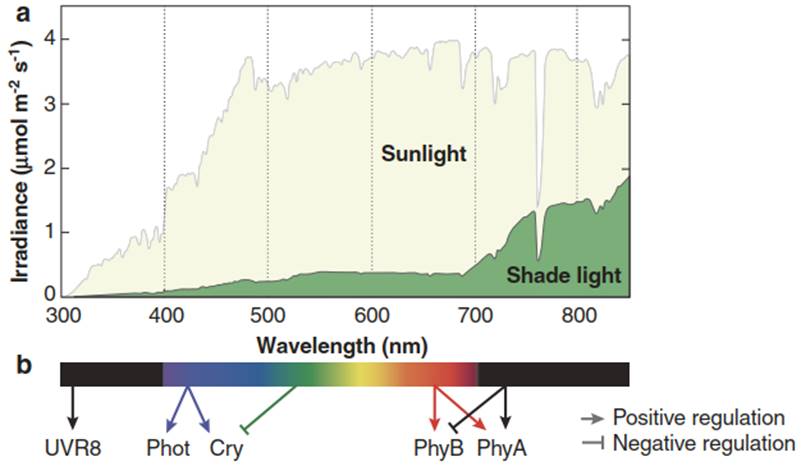
Fig. 3.18. Spectrum of visible light and the respective photoreceptors of terrestrial plants. Perception of shade light signals by photosensory receptors. a Spectral photon distribution of sunlight and shade light. b Impact of different wavebands on the status of phytochromes, cryptochromes, phototropins and UVR8 (Casal 2013)
Phytochromes. Phytochromes are proteins with an open-chain tetrapyrrole as a chromophore that changes its configuration upon absorption of red or far-red light. The effective ratio of red to far-red, 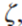 , refers to the broad spectral peaks of phytochrome in the red and far-red and can be calculated by Eq. 3.1 (Chelle et al. 2007):
, refers to the broad spectral peaks of phytochrome in the red and far-red and can be calculated by Eq. 3.1 (Chelle et al. 2007):
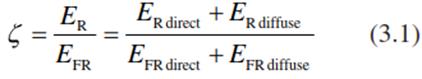
where ER and EFR are the spectral irradiances from the sun and the sky and the complex radiative transfer reactions within the canopy. ER comprises the radiation between 655 and 665 nm and accordingly EFR comprises that between 725 and 735 nm. Whereas 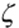 of the sunlight is around 1.2, it decreases in the understorey space to less than 0.2. Reactions that are triggered by (red) light (e.g. light-dependent seed germination) thus do not take place in the shade of a dense canopy. The situation changes in forest gaps where, because of the direct irradiation,
of the sunlight is around 1.2, it decreases in the understorey space to less than 0.2. Reactions that are triggered by (red) light (e.g. light-dependent seed germination) thus do not take place in the shade of a dense canopy. The situation changes in forest gaps where, because of the direct irradiation,  is high and seeds of pioneers can germinate rapidly. In contrast, the time span of sunflecks is too short to initiate red light-triggered morphogenic reactions, because the activation of phytochrome is reversible by a rapid return to low
is high and seeds of pioneers can germinate rapidly. In contrast, the time span of sunflecks is too short to initiate red light-triggered morphogenic reactions, because the activation of phytochrome is reversible by a rapid return to low  .
.
The scheme in Fig. 3.19 shows two phytochromes (A and B). Phytochrome B represents several phytochromes (B through E), which react in the same way and have been identified from several plant sources. While the interconversion of the physiologically inactive form (“Pr” for Pred) to the active form (“Pfr” for Pfar-red) by respective irradiation follows the same mechanism in all phytochromes, the inactivation mechanisms for Pfr differ. PhyAfr inhibits its own synthesis, and irradiation with red light as well as with blue light triggers its degradation. Synthesis and degradation play a secondary role in PhyB biochemistry as compared with photoconversion and slow reversion in the dark (Fig. 3.19).
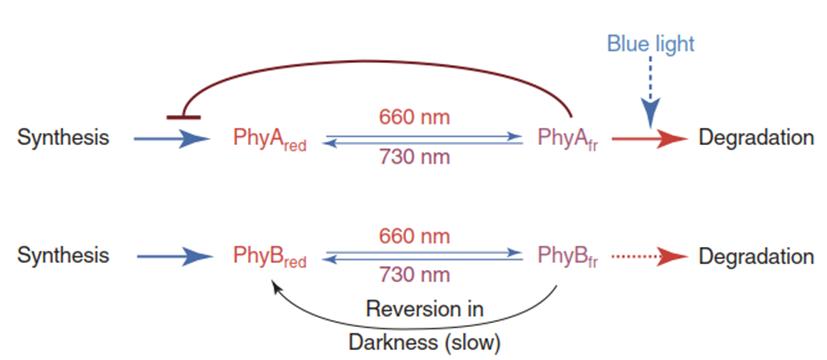
Fig. 3.19. The red-light Blue light switch mediated by phytochrome. Phyfr is the active form of all phytochromes. (Modified from Weiler and Nover (2008))
Note that photoconversion of phytochromes is associated with transport into (Pfr) and out of (Pr) the nucleus. The existence of a photoreceptor in two different states with distinct absorption maxima allows the monitoring of spectral quality (similar to colour vision in animals). Sunlight comprises both red and far-red light. Hence, the ratio of red to far-red light is crucial for the concentration of Pfr and the formation of a cellular signal.
Cryptochromes. Three cryptochromes have been found in A. thaliana, two acting primarily in the nucleus and one (Cry3) in mitochondria and chloroplasts. Cry3 is a photolyase with potentially some cryptochrome activity too (Liu et al. 2011). Cryptochrome 1 is the photoreceptor for high blue light intensity, while cryptochrome 2 is a sensitive blue light receptor that reacts at low fluence rates. The photoactive domains of the cryptochromes are homologous to those of the photolyases (N-terminal photolyase-homologous region (PHR)). However, because of their C-terminal extensions, cryptochromes do not exhibit photolyase activity (Fig. 3.20) but instead act as kinases.
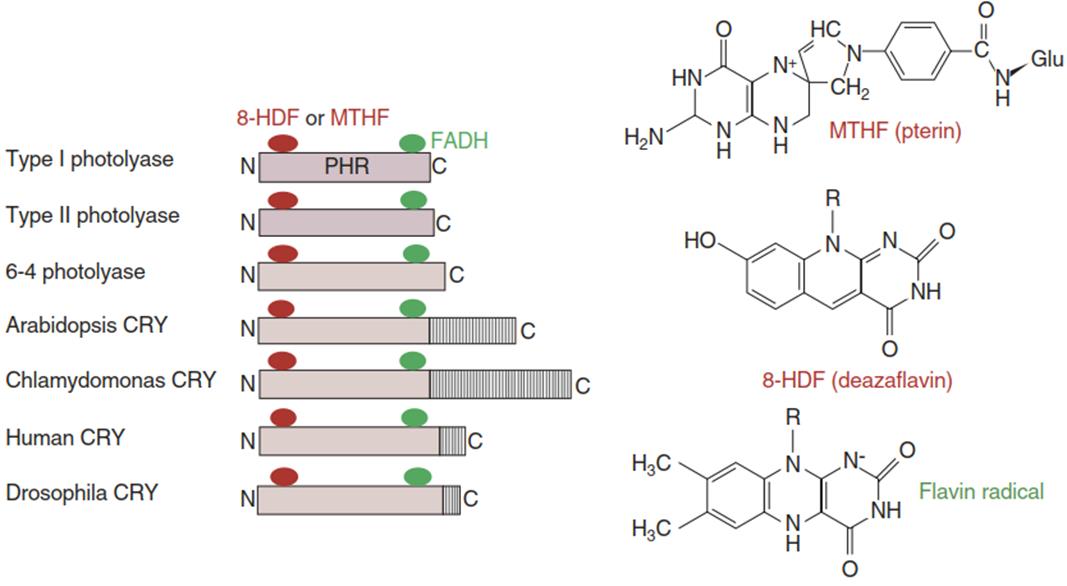
Fig. 3.20. Cryptochromes are evolutionarily derived from ultraviolet (UV)-activated photolyases involved in DNA damage repair. Photolyases (type I: Escherichia coli; type II and 6-4 photolyase: Arabidopsis thaliana), their substrates and co-substrates. A blue-light-triggered intrinsic energy transfer from pteridine to reduced flavin adenine dinucleotide (FADH) leads to an energisation of the pyrimidine residues of the dimers (in a DNA strand) and to a rearrangement of the carbon bonds, resulting in the cleavage of the dimers and restoration of the original DNA helix. Structures of pterin (MTHF), deazaflavin (8-HDF) and the intermediate flavin radical (FADH-). (Modified from Cashmore et al. (1999))
The photochemical mechanism(s) of cryptochrome light activation are not yet completely understood and the mechanisms of photoactivation and inactivation of both cryptochromes may differ to some extent (Li et al. 2011; Liu et al. 2011). Binding of the chromophores (5,10-methenyltetrahydrofolate (MTHF)) and FAD to the protein is non-covalent and the formation of an FAD radical anion (by electron transfer from MTHF) and a reduced FAD (FADH) radical (by subsequent proton transfer from the protein) appears obligatory in both cryptochromes. For activity, A. thaliana cryptochromes have to be phosphorylated. Upon additional (auto)phosphorylation after exposure to blue light, Cry2 (but not Cry1) is ubiquitinated and degraded. This mode of action resembles the situation in the phytochrome systems where PhyA is readily degraded, while PhyB is not (Liu et al. 2011).
Phototropins. Phototropins (Phot 1 and Phot 2 in A. thaliana) are blue light receptors of the plasma membrane with two flavin mononucleotides (FMNs) aschromophores. In darkness, their C-terminal protein kinase domain is sterically inhibited. Absorption of blue light changes the protein conformation, leads to dissociation from the plasma membrane and unlocks the kinase activity. In the activated state, the FMN becomes covalently bound to a cysteine residue of the protein chain.
This state of the protein is unstable and is readily reversed in the dark, whereupon the covalent bond breaks up. Along with cryptochromes and phytochromes, phototropins allow plants to respond to their light environment. For instance, they are important for the opening of stomata and mediate phototropic responses, e.g. the movements of chloroplasts (Fig. 3.4). Phot 1 is required for blue light-mediated transcript destabilisation of specific messenger RNAs (mRNAs) in the cell. For detailed chemical structures of the photoreceptors and mechanisms of gene regulation by light, see plant biochemistry textbooks (e.g. Buchanan et al. 2015).
Date added: 2025-01-17; views: 380;
