Sample Preparation Techniques. Filtration
One of the most common methods used for the pre-fractionation and pre-concentration of nanomaterials prior to characterization is membrane filtration. Straining is the dominant mechanism in membrane filtration. Generally, nanomaterials larger than the membrane pore size cannot pass through the smaller pore size membrane. However, when nanomaterial size is near the pore size of the membrane only a fraction of the nanomaterial will be filtered resulting in partial filtration. Partial filtration is caused by the variability of the membrane pore size, nonspherical shape of the nanomaterials, especially nanomaterials present in natural waters have shape characteristics significantly different (rods and plates) and other interaction such as electrostatic repulsion.
There are several configurations of the filtration process (Figure 3). Dead-end filtration, where the sample is pushed through the membrane and solids accumulate on the membrane surface, often causing so-called caking, leading to a reduction in the flow of liquid passing through the membrane, until the membrane is completely or partially clogged. Nanomaterials, smaller than the membrane pore size, will initially pass through the membrane but will be retained as caking takes place. Alternatively, cross-flow (tangential) filtration is a continuous process, where a constant flow is applied parallel to the membrane surface, preventing the accumulation of solids on the membrane surface.
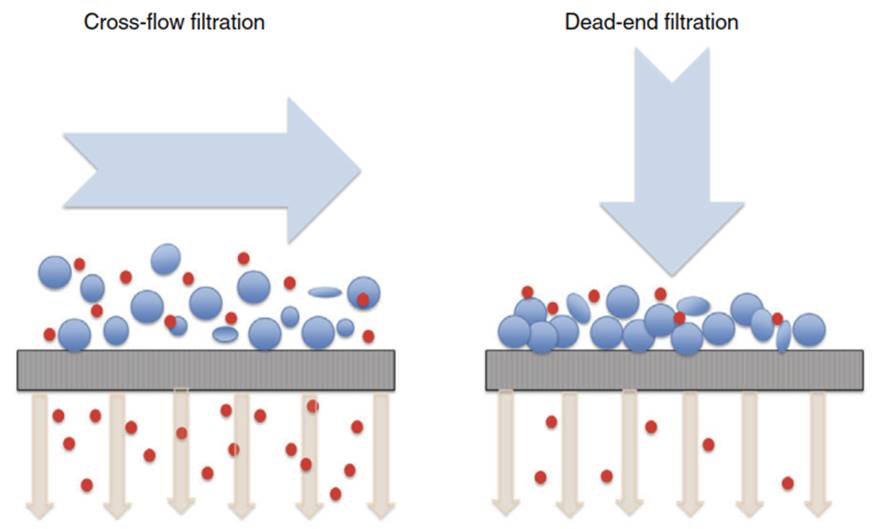
Figure 3. Schematic of membrane filtration principles
Improved size resolution can be achieved through multi-stage filtration using membranes with different cut off sizes, and then analyzing each fraction. In order to improve separation of nanomaterials, membranes with pore sizes of a few kDa (ultrafiltration) can be used which may effectively separate nanomaterials from dissolved constituents. In this case, a centrifugal force is often applied to overcome the high back pressure induced by the small membrane pore size. Particles deposited on the filter can be resuspended in deionized water and analyzed.
The main disadvantage of this technique is that the ultrafiltration membranes are calibrated against standards of a certain molecular weight, thus the pore size is not accurately known. Furthermore, these membranes have been found to be highly absorptive to metal cations. Electrostatic interactions may also prevent the nanomaterial from entering the membrane pores even if the physical size would permit passage.
Date added: 2025-02-13; views: 325;
