Sample Preparation for the Analysis of Nanomaterials in Water. General Aspects and Concepts
General Aspects and Concepts. Nanomaterial concentrations in natural aquatic systems are expected to be in the range of µgL-1 or lower. Generally, this concentration range is far below the concentration of the particulate fraction entity in natural water samples. The particulate fraction in these systems is a mix of constituents that vary in concentration, composition, size, and origin (natural/anthropogenic). Particulate and dissolved constituents, i.e. the sample matrix, often interfere with analytical equipment when attempting to detect nanomaterials in natural waters and most available analytical techniques cannot cope with both high matrix and low analyte concentrations. Hence, a progressive sequence of sample preparation steps is required prior to analysis.
This sample preparation method has to ensure compatibility between sample and analytical method. Because matrix and nanomaterial properties, composition, and concentration vary it is impossible to provide one general sample preparation method which is suitable to treat all matrix and nanomaterial combinations. The sample preparation method depends on the properties and concentration of the matrix and the target nanomaterials. Therefore, the sample preparation procedure has to be adjusted according to sample properties and analytical needs. Generally, the analytical effort for sample preparation increases with increasing sample complexity (Figure 1).
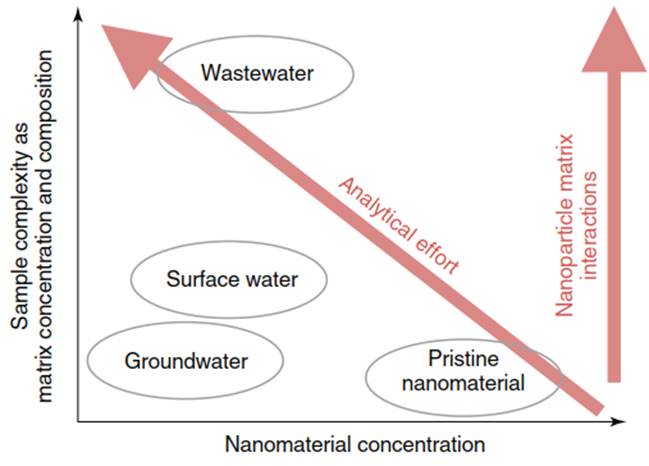
Figure 1. Comparison of analytical requirements for various environmental samples
The aim of sample preparation is to reduce complexity of the sample, to make the nanomaterial accessible to the analytical technique, and in some cases to increase nanomaterial concentration without altering their structure and composition. Alterations can be avoided using techniques that allow analysis within the sample matrix. This is noted as in situ analysis. In situ analysis of complex samples is only possible if the analytical technique is tolerant to the matrix, the matrix does not interfere with the nanomaterial analysis, and the nanomaterial is present in a detectable concentration. This may be the case for surface waters and groundwater with low suspended solids concentration.
However, for more complex matrices, for instance, wastewater effluent, separation of nanomaterials and matrix can be achieved either by taking advantage of differences in physical-chemical properties or by destruction of the particulate matrix. For example, physical properties, such as size, can be used to separate nanomaterials from larger particles by filtration. Alternatively, nanomaterials can be separated from the matrix based on density differences between the nanomaterial and the matrix, by sedimentation or centrifugation.
Another approach is based on matrix destruction, where nanomaterials remain in suspension while the matrix is removed by chemical or enzymatic clean up. This implies that nanomaterials survive the sample preparation procedure unaltered. In many cases, this can be achieved by a progressive stepwise sample preparation including homogenization, matrix removal/isolation, nanomaterial separation, and nanomaterial stabilization (Figure 2).
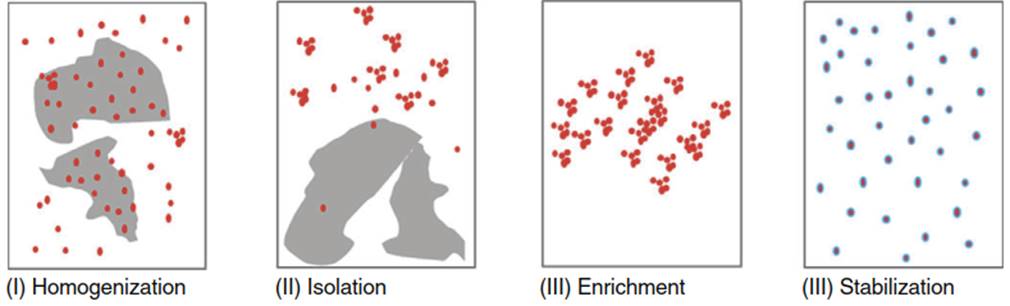
Figure 2. Stepwise sample preparation scheme for separation of nanomaterials from complex matrices
The number of sample preparation steps depends on the type of sample. For instance, when nanomaterial concentrations are below limit of detection of the analytical technique, enrichment and stabilization are necessary. In this article, we describe sample preparation techniques for matrix removal including filtration, centrifugation, and continuous particle fractionation and we explain sample preparation techniques for matrix removal including chemical and enzymatic clean up. Subsequently, we discuss sample preparation methods for fresh, high salinity, and organic-rich waters aiming for particle identification, sizing, and particle concentration analysis.
Date added: 2025-02-13; views: 321;
