Continuous Particle Size Fractionation Techniques
Particle size fractionation techniques were developed to reduce polydispersity and complexity of the sample prior to analysis. Field-flow fractionation (FFF) and hydrodynamic chromatography (HDC) are two continuous particle size fractionation techniques frequently applied for particles in water.
Field Flow Fractionation. FFF was originally developed for proteins and polymers, and it has been applied to fractionate natural colloids according to their diffusion coefficient. Depending on the fractionation principle, FFF techniques are classified as sedimentation, flow, thermal, electrical, and acoustical. Flow and sedimentation FFF are the most commonly used for characterization of natural colloids and nanomaterials in aquatic systems. Both employ a parabolic laminar flow profile in a well-defined channel. Particle size fractionation is achieved by interaction of particles with an external field, which in flow FFF is an eluent flow perpendicular to the laminar flow profile and in sedimentation FFF it is an enhanced gravitational force (Figure 5a).
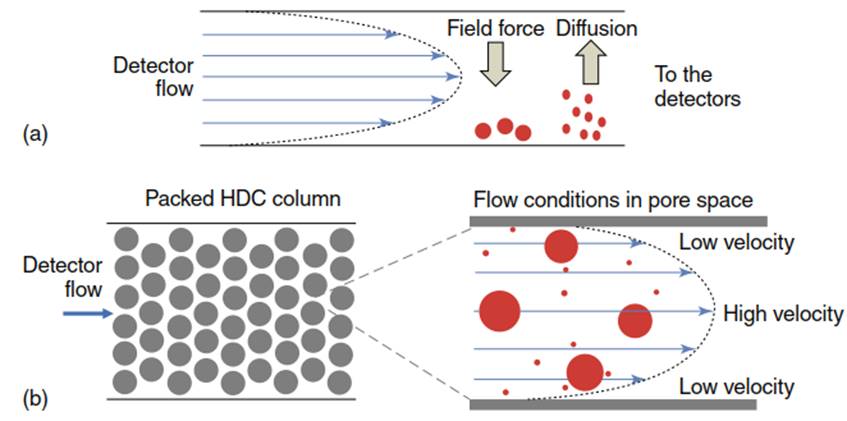
Figure 5. Schemes of (a) cross section of an FFF channel with separation principal (field force can be cross-flow in flow FFF or gravitational force in sedimentation FFF, and (b) hydrodynamic chromatography column and pore space
According to their diffusion coefficient, particles diffuse against the external field into the channel. Consequently, they are distributed nonuniformly along the laminar flow profile and smaller particles with higher diffusion coefficient experience a higher laminar flow velocity compared to larger particles and are thus transported faster. Further, details on FFF separation such as separation theory and particle sizing have been presented in a number of books and review articles.
FFF systems resemble liquid chromatography instrumental setups with the difference that particle fractionation is achieved in an open channel without a stationary phase and a separation column is not needed. As a continuous particle fractionation technique and in combination with elemental detectors such as inductively coupled plasma-mass spectrometry (ICP-MS) and optical detectors such as multi-angle light scattering (MALS), FFF has been recognized as a versatile and valuable tool to characterize natural colloids and engineered nanomaterials in aquatic environments. It outcompetes limitations which are commonly observed in filtration and centrifugation techniques (e.g. accuracy, resolution, quantification). Coupling the FFF to an element-specific detector allows determining (quantifying) the elemental distribution across a continuum of particle sizes. However, a tedious method development procedure is required prior to its implementation.
FFF application limits regarding sample properties are (i) large particles which may pre-elute due to steric inversion, (ii) matrix components that may influence the particle - membrane interaction, (iii) plate-like particles which may deposit onto the membrane surface, and (iv) polydispersity of particle suspension. In particular, particle-membrane interactions are often responsible for altering the particle elution behavior. The FFF techniques allow particle sizes of about 1 nm and 50 pm to be separated. However, precise particle sizing is not possible for the entire size range under constant separation conditions. The size resolution power that can be achieved with FFF is very high, compared to other techniques and lies in the lower nm range.
Flow Field-Flow Fractionation. In flow FFF, a secondary flow in the separation channel often named cross-flow is applied as the external field or separation force. This force is perpendicular to the axial parabolic laminar flow profile. The channel bottom is replaced by a permeable frit that is overlaid with a membrane of a defined size cutoff. The cross-flow passes through this membrane and particles larger than the cutoff remain in the channel. The membrane material and cutoff can be selected according to the particle properties, e.g. size and surface charge. Two sub-techniques have been developed, namely symmetric and asymmetric flow FFF. In asymmetric flow FFF, the cross-flow and the carrier flow are provided by one pump, and the cross-flow is controlled by a flow valve behind the channel. In symmetric flow FFF, the cross-flow enters from the channel top and is provided by a second pump.
Particle retention can be directly related to the hydrodynamic particle size via the Einstein equation. The hydrodynamic particle size is calculated based on channel dimension and crossflow rate. Alternatively, particle size can be determined based on calibration with particle size standards. In any case, for nonspherical particles, an equivalent hydrodynamic diameter of a sphere is calculated. However, particle size analysis by FFF can only be precise after thoroughly optimization of the FFF run conditions. The aim of the optimization process is to create run conditions where particles approach an ideal behavior, i.e. elution is solely controlled by the interaction between particle and external field. Correct sizing is hampered by particle aggregation, size specific as well as particle-type specific losses, particle membrane interactions, overloading, electrostatic repulsion from the membrane, and steric as well as entropic effects. These processes can be triggered by particle shape and particle surface properties such as charge. For example, plate-like particles are more prone to particle membrane interaction compared to spherical particles.
Sedimentation Field-Flow Fractionation. Another FFF sub-technique used for the separation of nanomaterials is sedimentation or centrifugal field-flow fractionation (SdFFF), where particles are separated not only based on their size but also their density. Separation follows the effective mass, or buoyant mass of particles and an equivalent volumetric diameter is obtained. In SdFFF, the separation force is generated by placing the channel along a rotating drum. The channel walls are solid and can be manufactured from nonpermeable inert materials like polycarbonate or polytetrafluoroethylene (PTFE). These materials have certain advantages compared to metal channels, such as resistance to solvents and lower metal background and therefore lower detection limits.
The injection procedure used for particle separation is called “stop flow procedure" (or “relaxation step procedure") where after injection the sample is allowed to equilibrate under the constant rotation speed. This allows particles to reach an equilibrium position which is important for obtaining high selectivity of the separation. Longer relaxation time is needed especially for low density nanomaterials. Following equilibrium, sample elution begins under the highest selected gravitational force, allowing the smallest particles to elute first. An exponential decay program balancing selectivity and separation time is then used to reduce the centrifugation force and allow larger particles to elute. Similarly to flow FFF, the outflow of the channel may be directed to various detectors.
The fractionation power of SdFFF is substantially higher than that of flow FFF and the method development is less tedious due to the absence of membranes. However, SdFFF is not as efficient on particles smaller than approximately 10 nm and long equilibration times may introduce artefacts (particles diffusing parallel to the channel).
Hydrodynamic Chromatography. HDC is a liquid chromatographic technique that separates nanomaterials based on their diffusion coefficients, which are inversely related to their hydrodynamic diameters through the Stokes-Einstein equation. The separation can be done in a tube without filling material or in a column packed with inert, nonporous beads that minimize particle-beads interactions and lead to improved sample recoveries.
The mechanism of separation lies in different positioning of particles in the parabolic flow profile. Depending on particle size particles experience different flow velocity during elution through the HDC column. The highest flow is in the middle of the tube and the lowest near the walls. Larger particles remain in the center of the flow profile and are moving faster through the column thus eluting before smaller particles (Figure 5b). The elution order is the same as in size exclusion chromatography (SEC); however, the elution mechanism is different. SEC uses porous particles column for separation and the pore size determines the exclusion limit.
HDC has been successfully applied to fractionate environmental colloids. Philippe and Schaumann demonstrated the characterization of Ag, TiO2, and ZnO particles in the presence of high organic matter contents by HDC coupled to UV-Vis, fluorescence, and ICP-MS. Particle shape was identified as a crucial factor for particle sizing by HDC. Proulx and Wilkinson highlighted the feasibility of using HDC for separation of complex mixture of nanomaterials in river water samples.
Comparison of Fractionation Techniques. Each of the separation methods mentioned earlier has certain advantages and disadvantages and is applicable in different size ranges (Figure 6). For example, HDC is faster and shows greater recoveries than FFF but has lower size resolution. The nonporous packing of the HDC columns (polystyrene microspheres) limits potential interactions with the sample demonstrating advantages over SEC where the adsorption of the sample to the packing material leads to sample loss and lower recovery rates. On one hand, the membrane-sample interactions may lead to low sample recoveries with FFF. Accordingly, a tedious procedure to optimize the carrier liquid composition (pH, ionic strength) and the cross-flow rate may be necessary to improve recoveries.
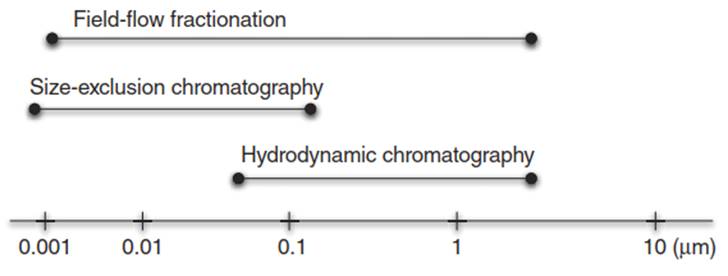
Figure 6. Applicable size range of the fractionation methods
On the other hand, the absence of a stationary phase in FFF allows more concentrated samples to be injected into the channel before overloading symptoms are observed. Furthermore, replacing a column is costlier than replacing a FFF membrane. While the HDC and SEC offer a large variety of column packing materials, the FFF choice of membranes is limited, but the FFF channels are compatible with many aqueous and organic solvents compare to column packing materials. In terms of the analysis time, it usually takes a relatively long time to setup and optimize a FFF method, while the analysis itself usually takes up to 90 min, depending on the technique and the application. SEC and HDC generally offer rapid separation typically <10 min. As with FFF, it is possible to couple HDC and SEC to multiple detection techniques (MALS, UV-Vis, fluorescence, ICPMS).
Date added: 2025-02-13; views: 319;
