Matrix Removal by Digestion or by Chemical Clean Up
Most analytical techniques are not compatible with complex matrices. Therefore, matrix has to be removed prior to nanomaterial analysis. In the case of inorganic nanomaterials, e.g. in a biological matrix, it is possible to treat the sample with an enzymatic and acidic or alkaline treatment. Organic matrices are commonly digested by nitric acid alone or in combination with hydrogen peroxide or hydrochloric acid. Samples are heated conventionally under atmospheric pressure or with microwave assistance under elevated pressure. Some nanomaterials, such as SiO2 and TiO2, may sustain this treatment, while others, such as Ag and CuO are degraded during. Therefore, different clean up reagents, such as enzymes are required in the latter cases.
Degradation or clean up of the matrix constituents by enzymes requires information about matrix constituents because specific enzymes are needed to degrade certain matrices. Generally, enzymatic clean up is used for biological matrices. For example, degradation of chicken meat requires an enzyme which is able to degrade proteins whereas plant material requires cellulose degrading proteins. A drawback of clean up procedures is the degradation of the particle surface by the clean up reagent. Very often alterations of the particle properties are observed. Organic coatings are transformed or degraded during this process. Conclusively, part of the information is lost. During digestion nanomaterials may be altered in terms of size, speciation, and surface chemistry. Therefore, stabilization of the remaining particle suspension is usually necessary to obtain reasonable particle size data.
In case, nanomaterial properties such as size are not required and the analysis aims at bulk concentrations, matrix and nanomaterials are digested together. There is no general digestion method which allows dissolving all variations of matrix nanomaterial combinations. For instance, TiO2 is insoluble to HNO3, whereas Ag is soluble. In many cases, a combination of nitric acid and hydrogen peroxide is used to remove the organic matrix. Inorganic constituents and most metals can be dissolved with aqua regia. Hydrofluoric acid is used to dissolve particles constituents made of TiO2, SiO2, or ZrO2.
Once matrix constituents and nanomaterials are completely dissolved, total elemental content of the sample can be determined, e.g. by ICP-MS. Total digestion followed by elemental analysis is only meaningful if the nanomaterial concentration is higher compared to the limit of quantification and the background signal. However, in most environments nanomaterial concentrations are low and mass concentration is often below the limit of quantification or the background signal.
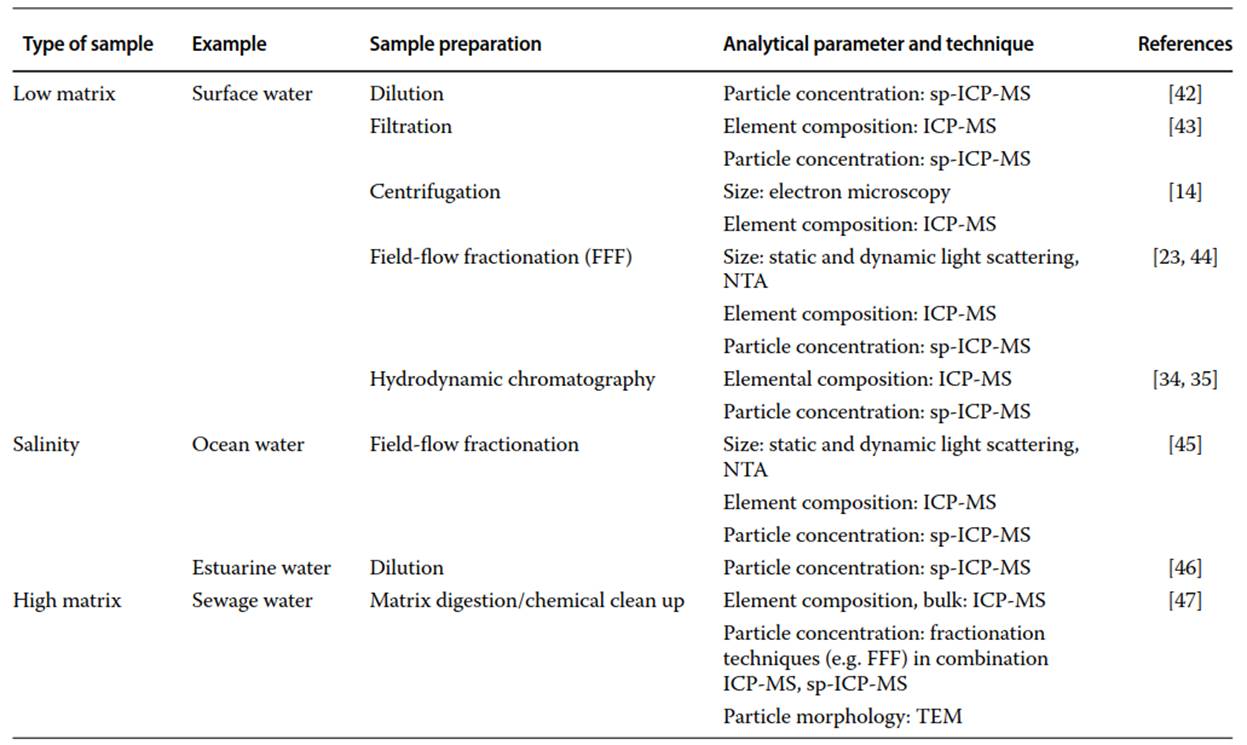
Table 1. Selection of sample preparation and analytical methods to analyze low and high matrix samples, as well as high salinity samples
Examples and Summary. Samples may have a low or high particulate matrix concentration. They can be either freshwater or high salinity water. In any case, sample preparation should be optimized toward a balance between reducing sample complexity and keeping nanomaterials intact. Here, we summarized sample preparation and assigned detection methods for each of the three types of sample (Table 1). Details on the analytical techniques including their limitations are presented in the article “Analytical techniques for nanomaterial characterization."
Date added: 2025-02-13; views: 329;
