Molecules, Morphology, and the Tree of Life: Bridging Perspectives in Phylogenetic Classification
In 1960, The Forest and the Sea, written by the accomplished naturalist Marston Bates, was published. It was his story of biology as told to the general public, largely by comparing the remarkable beauty and richness of life in a tropical rain forest and a tropical reef. This lovely book was an inspirational rallying cry to all biologists and aspiring biologists. But, you do not need to experience the splendor of a tropical rain forest or the Great Barrier Reef to appreciate that living forms are beautiful and astoundingly diverse. Just go for a walk in a local patch of forest, or a conservancy, or explore a trout stream. How do we interpret such a staggering array of shapes, sizes, and colors?
What attributes could an invisible bacterium possibly share with a whale or a giant sequoia tree some tens of orders of magnitude its size? Do they share common patterns in behavior or in survival strategies? How are the millions of species of microorganisms and macroorganisms categorized and compared meaningfully? They will be compared ecologically in this book, as will be to some extent the processes underlying the apparent patterns. But, first, we should pause to examine the big picture, namely, the broad diversity of life and what relationships or order underpin this seeming chaotic assortment of creatures.
The most biologically informative analytical grouping of organisms is based on evolutionary or ancestral relationships as determined by phylogenetic systematics (cladistics). Broadly speaking, the features showing heritable variation used to develop phylogenies can either be phenotypic (i.e., physiological, anatomical, or morphological, with fossils being the classic example) or genotypic (i.e., molecular, with nucleic acids, proteins, or chromosomes as examples) (Hillis et al. 1996). Which of these two phylogenetic approaches is the ‘better’ repository of information for determining a true historic genealogy? The respective merits of ‘morphology versus molecules’ as the gold standard, along with how the biological world would be categorized (below), were actively debated for many years. It became generally recognized that both types of data have inherent strengths and some weaknesses (see, e.g., Schopf et al. 1975; Brower et al. 1996; Raff 2007).
Either can be more or less suitable depending on the line of inquiry; ideally they are complementary. Hillis et al. (1996) summarized three fundamental requirements of any approach: (i) that the characters chosen should show appropriate levels of variation, together with (ii) a demonstrable and independent genetic basis, and that (iii) the data should be collected and analyzed in such a way that phylogenetic hypotheses can be tested meaningfully. In general, the modern molecular phylogenies have supported those based on morphology. Frequently, they have clarified, extended, or enabled the construction of accurate phylogenies where the morphological approach was impossible or misleading—a classic case is with the diverse truffle-like fungi (see, e.g., Bonito et al. 2013). It is also becoming increasingly common for organism phenotypic traits to be predicted from genotypic profiles (Costanzo et al. 2010; Ellison et al. 2011; Sunagawa et al. 2015).
Emerging from the ‘molecules versus morphology’ debate is whether cellular life is more appropriately organized into two (Prokaryota, Eukaryota; or possibly even Archaea and Bacteria; see below) or three (Archaea, Bacteria, Eukarya) major groupings, variously called empires, domains, or superkingdoms (Table 1.1). The informal division of the entire biological world broadly into prokaryotes versus eukaryotes based on physiological and morphological criteria was the traditional separation until molecular biologists discovered that prokaryotes were not monophyletic (i.e., all members did not share a common ancestor; Woese and Fox 1977).
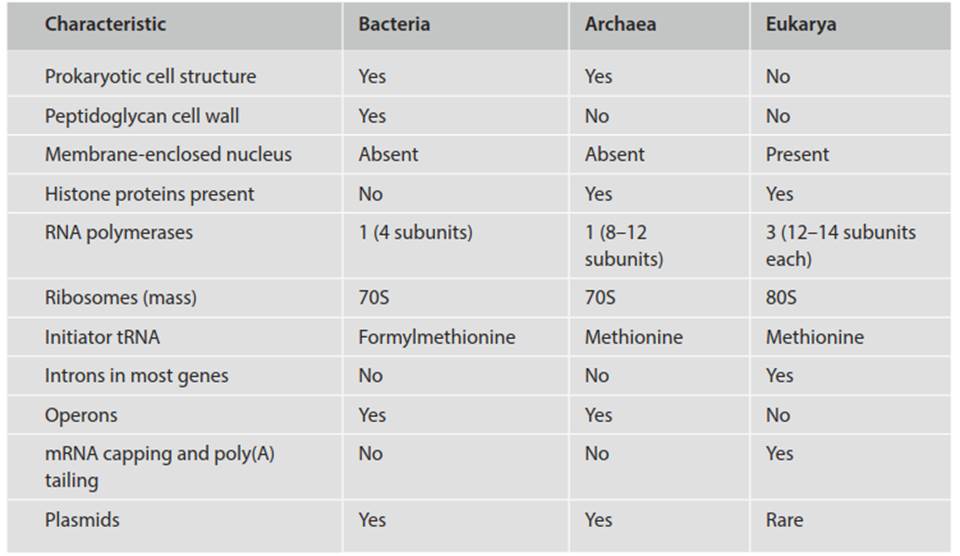
Table 1.1. Some contrasts among Bacteria, Archaea, and Eukarya (summarized from Madigan et al. 2015)
A further argument against the prokaryote/eukaryote divide was that categorization based on the absence of a structure (the nucleus) made little sense (Pace 2006, 2008). The counterpoint was that splitting prokaryotes into Archaea and Bacteria exaggerated their differences and placed too much emphasis on genotypic rather than phenotypic criteria (Mayr 1998; Cavalier-Smith 2006). While the prokaryotes generally are not morphologically ornate, two distinct evolutionary lineages are indeed evident and are now well accepted; the members of each are diverse genetically and metabolically.
In this text, for convenience and brevity, the term prokaryote is retained to refer informally and collectively to members of both domains Archaea and Bacteria. Such usage is in the same informal taxonomic sense that ‘invertebrate’ is a general term for any one of quite different organisms unified by the lack of a backbone. 'Prokaryote' is not being used in an evolutionary connotation in this book. Again, for reasons of brevity and simplicity, 'bacteria' with a small 'b' includes both bacteria and archaeons; when used with a capital 'B' and italicized, the word refers strictly to the evolutionarily distinct group Bacteria as opposed to Archaea.
Whether the so-called universal 'tree of life' is two or three domains, what can be said of their respective evolutionary origins? While a single-celled, bacterial-like, universal ancestor to all life commonly has been broadly assumed, how and when the more cytologically complex eukaryotes arose, and whether they are more closely related to Bacteria or Archaea remain highly contentious (7-Chap. 4 and Embley and Williams 2015). Actually, four possible scenarios are being debated (Mariscal and Doolittle 2015): (i) that the three branches—Archaea, Bacteria, and Eukarya—emerge more or less concurrently from a common primordial ancestor within a ‘community pool’ of pre-cell entities; (ii) that a Last Universal Common Ancestor (LUCA), i.e., the entity common to all life, is a relatively complex eukaryote-like form from which the sister clades Archaea and Bacteria subsequently diverge and become simplified, while the eukaryote branch evolves further complexity; (iii) that the tree is rooted on the branch leading to the Bacteria with further complexity arising after the Archaea and Eukarya diverge; and finally (iv) that the tree is rooted on the bacterial branch as in (iii) but the LUCA is a relatively complex entity as in (ii) from which the Archaea diverge and become simplified as do the Bacteria; i.e., both lineages become convergently prokaryotic.
Overall, educated opinions among these options vary, but recently the weight of evidence is shifting back to a two-domain primary clustering of life; however, those two primary domains may well be Archaea and Bacteria (Williams et al. 2013; Raymann et al. 2015). The eukaryotes arguably arose from the archaeal domain (Spang et al. 2015).
A phylogenetically based tree integrating all species would have to be based on molecular data (as supplemented by taxonomy where necessary, see below). This is because of several limitations in the applicability of morphological criteria to the Archaea and Bacteria. With some exceptions discussed in 7Chap. 4, there is in general relatively little fossil evidence for the prokaryotes. Even if there were, morphology is not very useful because of their frequently simple, uniform external structure (though, interestingly, evidence is emerging that they are internally complex; Graumann 2007).
Another limitation is that where species are compared over vast phylogenetic distances, few if any homologous morphological features exist. Differences in nucleotides within homologous (strictly, orthologous) genes among all known species provide a quantitative surrogate for conventional, morphologically based phylogenies. (Homologous, as used in the present sense, refers to a similarity derived from the same ancestral feature [phenotypic context], or to genes at the same locus in the genome [genotypic context]; orthologous refers to homologous genes that have diverged from each other because of separation of the species in which they are found [Barton et al. 2007]).
The tree in Fig. 1.1 shows a panoramic view of life as might be seen through the eyes of a molecular systematist. Indeed, the results of a massive phylogenic and taxonomic collaborative undertaking culminating in a comprehensive 'Open Tree of Life' currently containing 2.3 million tips (equivalent to ‘operational taxonomic units’; i.e., species where possible) have recently been published (Hinchliff et al. 2015).
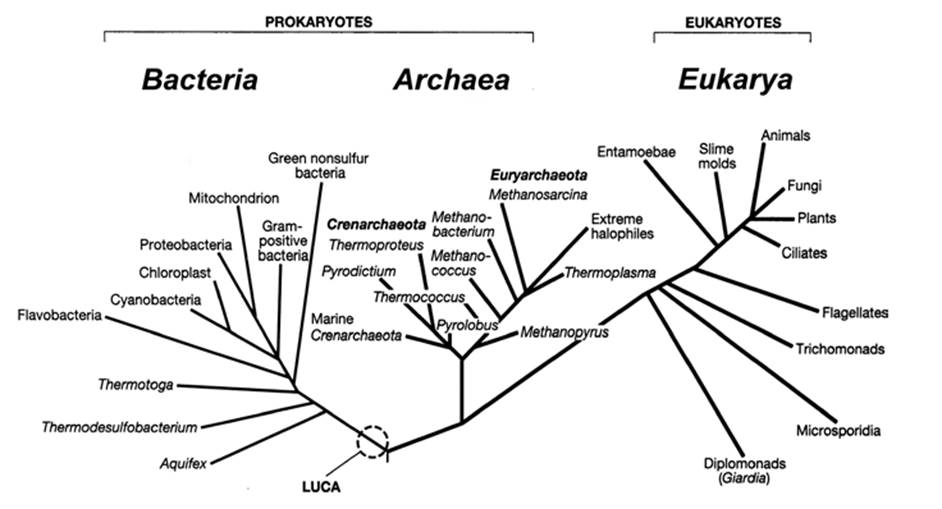
Fig. 1.1. A universal phylogenetic tree of life based on sequence variation in the rRNA gene showing three domains (Bacteria, Archaea, and Eukarya). The last universal common ancestor (LUCA) to all cellular life is shown here within the very early Bacteria domain and the tree implies that the Archaea and Eukarya are on a separate main lineage distinct from the one leading to the Bacteria. Position of groups remains in flux and numerous other phylogenetic hypotheses exist (see, e.g., Raymann et al. 2015; Spang et al. 2015). Reproduced from: Madigan, Michael T.; Martinko, John M.; Stahl, David A.; Clark, David P. Brock Biology of Microorganisms, 73th Ed. ©2012. Printed and electronically reproduced by permission of Pearson Education, Inc. New York, New York
Given rapid breakthroughs in molecular biology of recent decades, and in the mathematical algorithms used to create the trees, molecular phylogenies are beguiling in their apparent authority and simplicity. Nevertheless, it should not be forgotten that all trees, whether morphologically- or molecularly based, are basically hypotheses and interpreting them correctly is not trivial. The complexities and hidden assumptions in making phylogenetic inferences need to be understood (see Sidebar, below).
Now that the era of molecular systematics is firmly established, it is important to periodically remind ourselves about the distinctions between genotype and phenotype and what these mean in interpretations of species biology and species relationships, past and present. At the outset of this new era, Lewontin said in his treatise on evolutionary genetics (1974, p. 20) that “to concentrate only on genetic change, without attempting to relate it to the kinds of physiological, morphogenetic, and behavioral evolution that are manifest in the fossil record and in the diversity of extant organisms and communities, is to forget entirely what it is we are trying to explain in the first place.” A reasonable compromise is to recognize, as has Harold (1990) in his authoritative inquiry into the origin of form in microorganisms, that traits and especially complex traits such as morphology, are linked only indirectly to genes and that a real understanding of form can only be made at a much higher plane, orders of magnitude above the genetic. Multiple genes, multiple pathways, epigenetic factors, and the rules of biochemistry and physics are involved. This also is the thesis of Stewart (1998), who argues that the other, largely overlooked, secret to life is mathematics, which shapes what the genes can do: he emphasizes that genes at best are a recipe (not a blueprint) for life, a necessary but insufficient ingredient.
Date added: 2025-06-15; views: 196;
