Semiconductors, Elemental. Description
Elemental semiconductors may be classified either as intrinsic or extrinsic. An intrinsic semiconductor is one that exists in pure crystalline form, its conductivity depending entirely upon its intrinsic properties. An extrinsic semiconductor is one containing impurity atoms within its crystalline structure.
The addition of minute quantities of impurities (dopants) greatly affects the electrical properties of semiconductors, and they are therefore described as being structure sensitive. Because semiconductor materials only naturally occur in an extrinsic state, their value has largely depended upon developing techniques of refinement in order to make efficient use of their electrical properties.
An important factor in the selection of a semiconductor material is that its characteristics strongly depend upon the structure of the energy bands occupied by electrons. The band gap for a semiconductor is the energy gap between the valence (ground state) band and the conduction band (Figure 4).
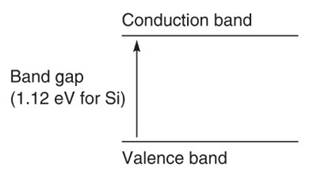
Figure 4. Electrons are raised from the valence band to the conduction band provided they have enough energy to jump the gap. Charge conduction is therefore directly related to band gap width
Materials with wide band gaps such as carbon in the form of diamond (band-gap energy 7 electron volts) may offer in theory the prospect of very high temperature operation since its wide band-gap means much higher energies are required to change the conducting state (other semiconductors change state at high voltage or high power). However diamond has so far remained virtually unused in electronic devices, due to practical difficulties in fabrication.
Those semiconductors with narrow band gaps such as grey tin (0.1 electron volts) have also so far been found to be unsuitable. The principal elemental semiconductors used in device manufacture to date have been the group IV elements germanium (Gi) (0.72 electron volts) and silicon (Si) (1.12 electron volts), the material being initially purified to an intrinsic state and controlled amounts of dopants then added in order to meet the desired specifications.
The first successful application of elemental semiconductor materials was as point contact rectifiers for the detection of electromagnetic waves in the early twentieth century (see Radio Receivers, Crystal Detectors). From 1900 to 1910 a wide range of elements and compounds were investigated, lead sulfide (PbS) and silicon proving the most efficient. Although the characteristics of these devices were substantially improved over the following years, this was almost entirely as a result of empirical affect.
Nevertheless, a variety of theories were put forward in an effort to explain the phenomenon of rectification. Early explanations included, for example, the thermoelectric effect (already rejected by Karl Ferdinand Braun), the electrostatic barrier theory, propounded by M. Huizinga (Germany) in 1920, and the cold emission theory of G. Hoffman (Germany) in 1921. About this time, Walter Schottky (Germany) also put forward a semiconductor theory with blocking layers and potential thresholds, but did not yet realize that holes would act as charge carriers. However, none proved satisfactory prior to the band theory of electronic conduction in solids, developed by Alan H Wilson (U.K.) in 1931.
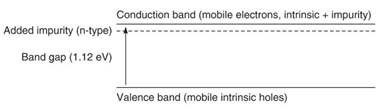
Figure 5. By adding minute controlled amounts of certain impurities to, for example, intrinsic silicon (group IV in the periodic table), the conductivity may be varied over a wide range. With the addition of и-type impurities (group V in the periodic table), electrons now become the majority carriers as the и-type impurity level donates electrons to the conduction band. Little energy is required due to the proximity of the impurity and conduction bands (0.05 electron-volt gap)
By the beginning of World War II a theoretical picture was beginning to emerge that explained the movement of charge carriers in semiconductor junctions. B. Davydov (USSR) produced the first model of a p-n junction in 1938, which included the concept of minority carriers (holders) in the semiconductor conduction. This was followed by an explanation of matter and to semiconductor junctions by Shottky in 1939.
A major problem facing the early investigators was their lack of appreciation of the extremely high degree of purity required in order to control the equilibrium concentration of mobile charge carriers within semiconducting material, and hence keep its resistivity correct. Consequently, experimental results were unpredictable and difficult to evaluate. In any case, no suitable process of refining semiconductor material to the level of purity required was then available.
A further important factor delaying progress was that, due to the overwhelming success of the thermionic valve, little stimulus existed until almost the end of the 1930s to carry out investigations into the properties of semiconductor materials. Gordon Teal (U.S.), recalling his doctoral thesis on germanium during that decade remarked, ‘‘Its complete uselessness fascinated and challenged me.’’
The urgent need for efficient ultrahigh frequency detectors for radar applications at the outbreak of World War II stimulated renewed interest in crystal detectors within the major advanced industrial countries. Isolated effort by individual researchers was now supplanted by the power of large research establishments and commercial corporations, with major emphasis being placed on the production of silicon and germanium point-contact diodes. In Britain, by 1940, the first commercially produced silicon diodes for use in radar frequencies were being made at Thomas Houston Limited, using polycrystalline silicon of 98 percent purity.
Later, crystals were grown at the General Electric Company from highly purified silicon powder, to which had been added controlled fractions of aluminum and beryllium. Although great efforts were now being made in material preparation, the work largely proceeded on an ad hoc basis.
After 1941, the center of attention of semiconductor materials research shifted to America and to Bell Laboratories in particular. One early wartime method of material preparation was to produce pure germanium and silicon films by pyrolitic deposition on to tantalum filaments. It was then used to manufacture infrared detectors and photoconductors. The technique of material purification by the segregation of impurities through the repeated freezing was also employed at this time.
From February 1942 onwards, germanium microwave diodes were being manufactured in quantity at various establishments and a basic research program on germanium was instituted. Pennsylvania University concentrated on silicon, and cooperating closely with industry, was soon producing material with a spectroscopic purity better than 99.9 percent using a process involving the reduction of silicon tetrachloride with zinc. Other firms, including Sperry, were also manufacturing microwave diodes.

Figure 6. With the addition of p-type impurities (group III in the periodic table), holes now become the majority carriers as electrons are raised from the valence band to the p-type impurity band. Little energy is required due to the proximity of the impurity and valence bands (0.05 electron-volt gap)
Throughout the period of the war, polycrystalline material was used exclusively in the manufacture of semiconductor devices. However, in October 1948, Gordon Teal and John Little (Bell Laboratories) grew the first single germanium crystals by a crystal-pulling process developed initially by Jan Czochalski in 1918. Within months, crystals were being doped with impurities to produce germanium ‘‘grown junction’’ diodes.
The period 1952-1953 was particularly important in terms of material purification and the consequent evaluation of the physical properties of semiconducting materials. The process of zone refining was introduced by Willian Pfann in 1952, and float-zone refining by Henry C Theurer in 1953. From then on, it was possible to produce material of a consistently high quality.
In 1952 Teal and Ernest Buehler also produced large high-quality silicon crystals by pulling from the melt, which was impurity doped to form single crystal diodes. With such high purity material available, much more accurate measurements of electron and hole carrier mobility could now be carried out, consequently achieving a deeper understanding of the properties of intrinsic semiconductors.
In 1954, silicon grown-junction transistors, with a high degree of lattice orientation, were first fabricated by Gordon Teal at Texas Instruments, using a crystal-pulling process. The ability of silicon (due to its wider band gap) to operate at higher temperatures than germanium was of particular importance. Although methods of refining semiconductor materials have since been greatly improved, progress has been of a steady, incremental nature.
The technique of growing everlarger silicon crystals on a mass production basis, free from dislocations and other defects, has also constantly advanced. In 1975, crystal diameters were typically around 50 to 75 millimeters. By the end of the century, diameters had risen to 300 millimeters. From the mid-1960s onwards, silicon has almost entirely replaced germanium in device manufacture, a major factor having been the ability to grow an electrically stable oxide on its surface, thus rendering it suitable for planar fabrication.
The group VI element selenium (Se) has played an important role in the fabrication of electrical and electronic devices. From 1931 onwards, selenium rectifiers were used commercially on an increasing scale. The first practical solar cells, using selenium, were constructed as early as 1883. Their conversion efficiency was very low (less than 1 percent) and further development was neglected until the early 1930s, when they were introduced commercially.
Cheap to produce, selenium photovoltaic cells were then employed in an increasingly wide range of applications, including photographic work. This use was aided by a peak spectral response (556 nanometers) approximating to that of the human eye. However, when used as photo detectors, conversion efficiencies still remained at about 1 percent and response time was limited. A significant breakthrough came with the development of the silicon photovoltaic cell by Russell Ohl (U.S.) in 1941.
By 1944, silicon photovoltaic cells with conversion efficiencies of 6 percent were being manufactured, this figure rising to about 12 percent by 1960 and to about 16 percent by 1996. Silicon photocells have therefore replaced selenium, being more efficient, mechanically robust, and cheaper to manufacture.
The group IV element carbon (C) has also been widely used within the radio and electronics industry. Apart from possessing a negative temperature coefficient, it has the property of decreasing its resistance under applied pressure. This effect was utilized in the invention of the carbon microphone by Thomas A. Edison (U.S.) in 1877. A transverse-current carbon microphone, developed in Germany by G. Neumann in 1924, was used by the BBC between 1926 and 1935.
Carbon-film resistors were first made by T.E. Gambrell and A.F. Harris (U.K.) in 1897. The high-stability cracked-carbon type was invented at Siemens and Halske (1925) and the sprayed metal-film type a year later by S. Lowe (Germany). Other uses include automatic voltage regulators, electrodes, and brushes in the electrical machinery.
Date added: 2024-03-05; views: 679;
