Potential Treatments and Therapies
There are three main treatment strategies being pursued for HD and ALS, drugs, stem cell therapies and genetic manipulation. The most conventional is drug screening for small molecules that will either delay the progression of the disease or ameliorate the symptoms of the disease. Several experimental drugs have been tried including Rapamycin in HD and Minocycline in ALS and HD (Ravikumar et al., 2006; Berger et al., 2006; Van Den Bosch et al., 2002; Plane et al., 2010).
These drugs have side effects that have made them undesirable for long-term treatment. Rapamycin inhibits the TORC1 complex and has become a drug of interest for HD with the elucidation that the TORC1 complex of the mTOR pathway has been shown to interact with mutant HTT (Pryor et al., 2014). Unfortunately Rapamycin has side effects including diabetic-like symptoms and lung toxicity (Lamming et al., 2012; Pham et al., 2004; Chhajed et al., 2006).
Minocycline, a candidate drug for both HD and ALS was found to be ineffective in HD and exacerbated the progress of ALS, perhaps through increased immunosuppression (Gordon et al., 2007; Plane et al., 2010; Huntington Study Group, 2010). Drug screening for small molecules continues and there are several ongoing Phase II clinical trials for ALS and HD (Shefner et al., 2013).
As more information is discovered about HD and ALS more potential targets are revealed. The potential for stem cell therapies is being explored in both HD and ALS, in HD it has been shown in mice that injections of stem cells in the brain can have a beneficial effect on symptoms, for ALS there are ongoing human trials to evaluate the efficacy of this treatment in muscles (An et al., 2012; Sadan et al., 2009; Cohen, 2013).
The main challenge for these experiments is the successful genetic modification of the stem cells and the delivery of them to the appropriate local within the patient (Figure 3). The third strategy involving genetic modification has advanced dramatically in the last 5 years as newer and more reliable tools have emerged in the field. Several group of DNA modification enzymes (Zinc Fingers, TALEs, and CRISPR) have the ability to replace the mutant gene with a modified nonmutant copy of the gene, or to regulate transcription levels (An et al., 2012, 2014; Bailus and Segal, 2014; Segal and Meckler, 2013).
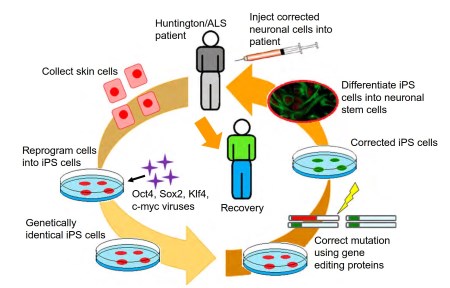
Figure 3. Use of induced pluripotent stem cells for therapeutics. Summarized are the steps for the use of induced pluripotent stem cells for autologous stem cell therapy in genetic diseases. (1) First skin cells are collected from patient; (2) then the cells are reprogramed into pluripotent stem cells; (3) the stem cells are genetically corrected to remove the disease mutation; (4) the induced pluripotent stem cell is converted into the relevant cell type needed in the disease; and (5) finally the cells are transplanted into the patient
The major challenge of this technology is efficiency and deliverability. Gene modifying tools are currently being utilized to modify stem cells, which are then injected into the patient in an effort to replace the damaged and dead cells with new nonmutant protein coding cells (An et al., 2014; Hou et al., 2013; Horii et al., 2013; Yin et al., 2014; Ye et al., 2014).
As more is understood about HD and ALS better therapies can be designed and delivered to patients in an effort toward increasing life span and survival rate in patients.
Date added: 2024-06-13; views: 441;
