The calcium homeostasis
The ions most responsible for creating and maintaining the electrochemical potential difference across the membrane are sodium and potassium. There are other ions in biological solutions, such as calcium and chloride (Table 3.2), which influence, modulate or change the potential difference of specific cell types. Ions such as calcium, magnesium, copper, iron and zinc are particularly important for cell function as activators of enzymes, as co-factors in protein synthesis or as stabilizers of nucleic acids.
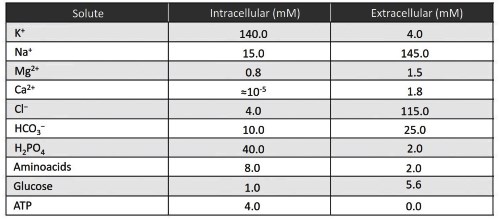
Table 3.2. Mean intracellular and extracellular concentrations of various solutes in a generic mammalian cell
Of all the ions mentioned, the only one that can perform a variety of functions in the cell, each with a particularly high level of effectiveness, is the calcium ion. Indeed, it has an electrogenic action on the membrane potential as a permeant ion and a cytoplasmic function as a second messenger.
Second messengers are molecules that transfer information within the cell and are activated by an appropriate primary stimulus, which may be electrical or come from the activation of a membrane receptor.
The primary stimulus rapidly increases the cytoplasmic concentration of the calcium ion, which transfers the message, amplifying it, within the cell to other molecules and activates specific functions, such as the activity of enzymes or the regulation of numerous intracellular enzyme cascades, or even the stimulation of the translation of several genes. Calcium is also solely responsible for the variation of the membrane potential in many types in the central nervous system, e.g., the lower olive, and in the hear cell t muscle.
The possibility of infinite modulation means that, as a second messenger, calcium also plays a key role in skeletal and cardiac muscle contraction, synaptic communication and the modulation of membrane channels (Figure 3.16).
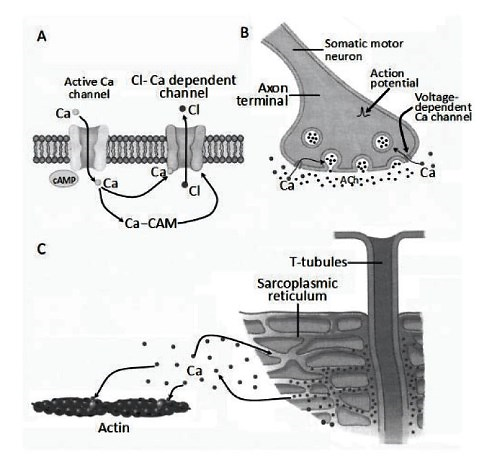
Figure 3.16. Examples of the role of the calcium ion (Ca) in cell physiology. The accumulation of intracellular calcium is essential (A) as a second messenger in numerous intracellular enzymatic cascades involving calmodulin (CAM) and cyclic adenosine monophosphate (cAMP) to activate, for example, calcium-dependent chloride channels, (B) for synaptic neurotransmitter release in the presence of the primary action potential stimulus and (C) to activate the contractile protein actin in the excitation- contraction coupling of skeletal and cardiac muscle
The primary stimulus, usually a molecule that binds to a specific calcium channel, rapidly increases the cytoplasmic concentration of calcium ions. Calcium transfers the message within the cell to other molecules: the message is amplified and activates specific functions, such as the opening of other ion channels like potassium or chloride.
In synaptic release, there is first an entry of calcium from the external environment very rapidly. The increase in calcium concentration activates, with relatively long timescales, its release from the intracellular stores.
In the muscular system, the primary stimulus consists of a change in the membrane potential due to the activity of sodium and potassium channels in skeletal muscle and calcium channels in cardiac muscle. Calcium is released from the sarcoplasmic reticulum stores and activates the contractile machinery.
Calcium’s function as a second messenger can be a slow process. Most of the time, enzyme synthesis is required. By permeating the plasma membrane through specific channels, the calcium ion can change its cytoplasmic concentration, especially in the micro-environment close to the membrane, contributing to calcium-dependent functions, such as regulating the function of membrane proteins, including calcium channels themselves.
At rest, the intracellular concentration of calcium ions is kept extremely low, in the order of 10-8 – 10-9 M (to be compared with 10-5 mM in Table 3.2). Calcium ions are highly reactive and concentrations between 10-6 M and 10-3 M, if maintained for long periods, are toxic to various cellular functions, as they can induce protein precipitation, block ATP synthesis, alter the cytoskeleton and activate calcium-dependent hydrolytic enzymes.
To ensure that the cell has a sufficient supply of calcium ions, they are actively sequestered in intracellular stores, a specialization of the endoplasmic reticulum, present in all cells. Calcium stores are able to accumulate the divalent ion by means of a calcium-dependent ATPase.
The calcium pump or Ca2+-dependent ATPase is located on the membrane of the stores, is always active and increases its calcium sequestration function in relation to the cytoplasmic concentration of the ion. Calcium concentration inside the stores can reach values of 10-2 to 10-3 M. Calcium is usually bound with low affinity to a protein, calsequestrin, to decrease its apparent concentration and thus facilitate the work of the pump.
When the cell receives an external or internal signal, which may be electrical, mechanical, chemical or hormonal, it is able, in a few tens of milliseconds, to increase the concentration of calcium ions, either diffusely throughout the cytoplasm or in subcellular micro-environments, to a value of 10-3 to 10-4 M.
This increase in concentration can occur in two precise ways: either by inward flux from outside through specific channels in the plasma membrane, or by release from intracellular stores, again through specific channels in the membrane of the endoplasmic reticulum. The external environment and intracellular stores can therefore be considered reserves of calcium ions and thus environments with a high level of chemical potential energy.
To prevent the high concentration of calcium ions from damaging the cell, there is usually an increase in cytoplasmic concentration following a stimulus in the form of calcium transients. The dynamic process involves the massive and instantaneous release of calcium from stores and its recapture by the Ca2+-dependent ATPase in an equally short time of a few tens of milliseconds.
However, there are actions promoted not only by different concentrations of cytoplasmic calcium, but also by the time that this divalent cation spends in the cytoplasm. Intracellular actions promoted by calcium release can last a few milliseconds, like in muscle contraction, or tens of minutes, for example during the fertilization process.
Because calcium cannot remain elevated in the cell for such a long time without being toxic, the solution used by the cell is to operate with very large repeated calcium transients, but with a duration of only a few milliseconds, which increase the intracellular concentration up to 10-3 M. Calcium-dependent reactions, which have longer timescales for their activation, 'sense' a cytoplasmic calcium concentration, which is the time integral of the transients.
In this way, the concentration becomes dependent on the frequency with which calcium is released from the stores. Different frequencies at which calcium transients are produced are used to control the concentration in a systematic way over time. The frequencies often coincide with the frequency of stimulation of the target cell.
Date added: 2024-07-10; views: 512;
