The movement of water. Aqueous pores. Water flows
Water must be able to move passively through cell membranes to compensate for the flow of salts and other substances, in order to maintain constant osmotic pressure. Water, like any other molecule, flows according to the concentration gradient. Water is a solvent, but from a chemical point of view, is like any other component present in the solution.
The concentration of water is less in the presence of a solute compared to pure water. Thus, if the solute at time = 0 retains the higher probability to diffuse towards pure water, water moves in the opposite direction with the aim of diluting the solution.
Aqueous pores. The plasma membrane can allow apolar molecules to pass through the phospholipid bilayer or through large aqueous protein pores or aqueous
pores with a diameter comparable to the diameter of the molecule to diffuse. For large pores, Equation 4.3 is valid. The diffusion coefficient D is that of free diffusion, the partition coefficient is equal to 1 and the area involved in diffusion is the total area of the pores Ap (Figure 4.5A). Thus, the permeability of the substance is

where Am and X are the area and thickness of the membrane, respectively.
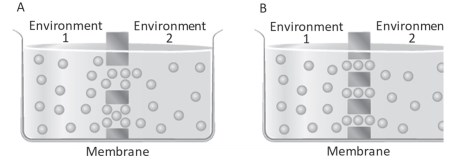
Figure 4.5. The flow through the large-pore (A) and small-pore (B) porous membrane
In the case of small-diameter pores, on the other hand, the net flux Jn can reach saturation, as within the pore, molecules can interact with the pore walls and with each other as they cross the membrane in a single row, so the two unidirectional fluxes are not independent (Figure 4.5B). The permeability coefficient is variable and can only be determined experimentally. Aqueous pores of small diameter include, for example, ion channels.
Water flows. Water molecules move if there are two phases with different concentrations of a given substance S. Such movements are always simple diffusion but can occur in different physiological conditions.
Suppose we have a cylinder similar to the one in Figure 4.3. Environment 1 contains distilled water and environment 2, distilled water with a solute, e.g., sucrose at a given concentration. The two environments are separated by a semi-permeable membrane, i.e., permeable to water but not to the solute (Figure 4.6A). The concentration of water in environment 1, Ca1, is greater than the concentration of water in environment 2, Ca2, because in the latter, each unit volume contains both water and solute molecules.
The individual water molecules pass through the phospholipid layer by a mechanism similar to the diffusion of an anelectrolyte. Equation 4.3 becomes:
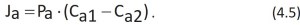
Ja is the net flux of water per unit area of the membrane. Pa is the permeability coefficient for water. Pa takes into account the mobility and the partition coefficient r of water in the membrane.
The passage of water from environment 1 to environment 2 is called osmosis, from the Greek osmos, to push. If environment 2 has rigid walls (Figure 4.6A), following the physical principle of liquid incompressibility, there is an increase in the water pressure at the membrane without a physical passage of water. If the top of environment 2 is mobile (Figure 4.6B), water flows from 1 to 2. Under these conditions, the water actually passes through the membrane, increasing the volume in 2.
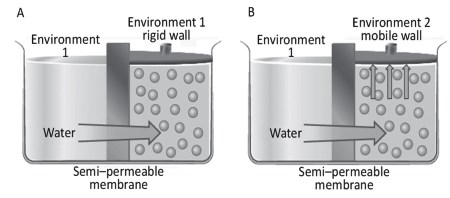
Figure 4.6. The diffusion of water in the presence of a semi-permeable membrane with rigid (A) and mobile (B) walls
Water flows until the difference in hydrostatic pressure between 1 and 2 (due to the greater weight of the water column in 2) counterbalances the net flow of water between 1 and 2. The difference in hydrostatic pressure that counterbalances the flow of water is the osmotic pressure. The osmotic pressure is therefore the pressure that can be measured as a net flow of water from an environment with a high concentration of water to one with a low concentration due to the presence of a solute.
This is referred to as the volumetric flow of water JV:
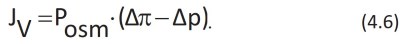
Δπ and Δp are the difference in osmotic pressure and hydraulic pressure, respectively, between environment 1 and environment 2. Posm is the osmotic permeability coefficient, which depends on the mobility of water and the partition coefficient for water between the membrane and environment 1. In steady state conditions, JV is = 0 in both cases: the osmotic pressures and hydraulic pressure are equal with an opposite sign.
Osmolarity. The osmolarity is the sum of the concentrations of all molecules and ions in solution regardless of whether they can cross the plasma membrane or not. A solution containing sucrose at a concentration of 1 mol/L (1 M solution) has an osmolarity of 1 Osm (Osmole), whereas a 1 M NaCl solution will have an osmolarity of 2 Osm, as the salt dissociates into ions and both the cations and anions contribute to the osmolarity.
The higher the osmolarity of a solution, the greater the osmotic pressure it exerts on water to diffuse through a semi-permeable membrane. The higher osmolarity means a higher concentration of particles and therefore a lower concentration of water.
This leads to a greater osmotic flow of water and therefore a greater hydrostatic pressure, i.e., a greater osmotic pressure to balance the system. If a solution is isosmotic with respect to another solution separated by a semipermeable membrane, the water flow is null; if it is hyperosmotic, water diffuses from the other solution; if it is hypoosmotic, water diffuses towards the other solution.
The osmolarity, therefore, although referring to the concentration of solutes, acts on water flows. In most living organisms, the solution inside the cell and the solution outside the cell are isosmotic with an osmolarity of about 300 mOsm (300 · 10-3 Osm).
Date added: 2024-07-10; views: 477;
