Counter-current transport
Consider the situation in which two tubes, T1 and T2, carry an aqueous solution of a salt (Figure 4.14). The solution of tube T1 reaches a constant, high concentration of 200 mM (Figures 4.14A and 4.14B, sector 1) because it comes from a large-volume reservoir, while the solution of tube T2 reaches a constant, low concentration of 80 mM (Figures 4.14A, sector 1; Figure 4.14B, sector 6) because it comes from a tissue that metabolizes the solute. The epithelia of T1 and T2 are semi-permeable, i.e., permeable to the solute but not to water.
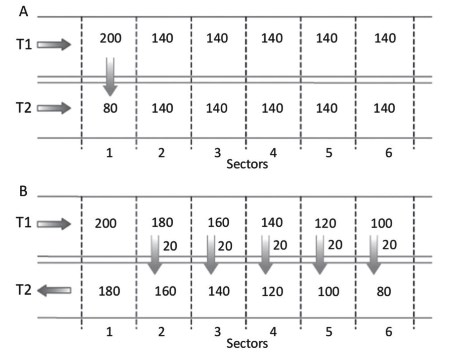
Figure 4.14. If the solutions in two parallel tubes flow in the same direction, there is a limited exchange of substances (A). If the two solutions flow in the opposite direction, the exchange of substances is much more effective (B). The numbers represent the solute concentrations in mol/L in the tubes and the quantities exchanged in moles at the downward arrows, which are purely illustrative
If the two solutions flow in the same direction (Figure 4.14A), the solute diffuses from T1 to T2 and the process goes on as the two solutions proceed in their respective tubes. The high concentration gradient induces a large solute flow and the rapid attainment, in the example of Figure 4.14A already in sector 2, of a stable equilibrium of concentration between the solution in T1 and the solution in T2, without exploiting the whole length of the tubes for the exchanges.
These exchanges are in limited quantity (the concentration of the solute in B increases by 60 mM), such that the solution in T1 exits the tube with a high concentration of solute (140 mM, Figure 4.14A, sector 6).
If, on the other hand, the two solutions flow in the opposite direction (Figure 4.14B), the solution in T2 acquires 100 mM from the solution in T1 using the whole length of the tubes for the exchanges and the solution in T1 exits the tube with a low solute concentration (100 mM, Figure 4.14B, sector 6). The concentration of solute in T1, in fact, decreases steadily from sector 1 to sector 6; the concentration in T2 increases steadily from sector 6 to sector 1.
This is because the solute passes in all sectors from T1 to T2 due to the establishment, along the whole length of the tubes, of a stable disequilibrium with a small constant concentration gradient at the level of each sector (in the example of Figure 4.14B of 20 mM), but sufficient to produce the necessary fluxes of solute from T1 to T2. There is a functional combination of solution flow rate and solute flow rate.
For example, at T1 in sector 4, a solution arrives in which the solute concentration has changed from 160 mM to 140 mM due to solute flux from T1 to T2, and at T2, a solution arrives with a solute concentration of 120 mM because, by moving from sector 5 to sector 4, it has acquired solute from T1. Extending this mechanism to all sectors, and remembering that concentration changes along the tubes are continuous, it becomes clear how small single gradients can produce a large solute flux.
Counter-current transport, a clear example of how structure and function are closely related, is present, as mentioned, in the gills of teleost fish. The gills of other species of fish and those of amphibian larvae are similar. Teleost gills are formed by 8 gill arches from which emerge, perpendicular to their longitudinal axis, two rows of flat filaments. These filaments in turn form, by eversion of the epithelium, a large number of lamellae arranged perpendicular to the surface of the flat filaments and transversal to their major axis (Figure 4.15B).
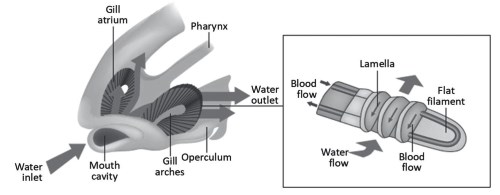
Figure 4.15. Diagram of the teleost gill apparatus (A) and structure of the gill arches (B)
Seawater with high pO2, partial pressure of dissolved oxygen, enters through the mouth cavity, flows forcefully along the lamellae from outside to inside (Figure 4.15B) and exits, after passing through the pharynx, the branchial arches and the branchial atria, through the opercula (Figure 4.15A). The blood, with low pO2, flows inside the gills in the opposite direction.
The situation described is similar to that in Figure 4.14B, substituting the concentrations for the partial pressures of oxygen: the flow of seawater corresponds to that of the solution in tube T1, although in this case the pO2 can be considered almost constant, given the large volume of solution and the high diffusion coefficient of gases in water, while the flow of blood corresponds to the solution in tube T2. The mechanism just described is so efficient that seawater can yield up to 80% of dissolved oxygen to the blood.
Date added: 2024-07-10; views: 381;
