Tonicity. Water transport
Let us consider a cell containing a solute Si that is not diffusible through the membrane, with a concentration that gives an osmolarity of 300 mOsm, immersed in a solution with an osmolarity equal to that internally and containing a molecule Se that diffuses freely (Figure 4.7A). In the two solutions, the water concentration is equal and therefore there is no flux, i.e., the two solutions are isosmotic.
The Se molecule, however, diffuses inside the cell, due to the presence of a concentration gradient, according to Equation 4.3; the osmolarity inside the cell increases because the water concentration decreases, so an inward flow water is established according to Equation 4.5 (Figure 4.7B).
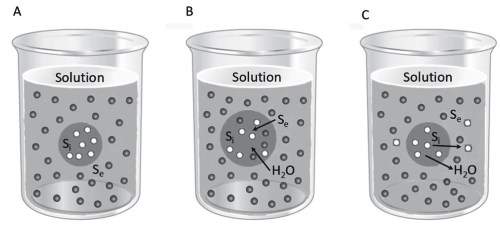
Figure 4.7. If Si (light dots) is non-diffusible and Se (dark dots) is diffusible (A), the cell becomes loaded with Se and water (B). If Si is diffusible and Se non-diffusible (A), the cell loses Si and water (C). In the first case, the external solution is hypotonic and in the second case, hypertonic
The cell swells until it bursts or until the elasticity of the membrane generates a level of hydrostatic pressure that blocks the inflow of water. The solution containing the Se molecule is hypotonic compared to that inside the cell. On the other hand, the solute inside the cell Si diffuses through the membrane, but the Se molecule does not, and the two solutions are still isosmotic: there will be flow of the solute Si towards the external environment, accompanied by water escaping from the cell. The cell reduces its volume and shrivels (Figure 4.7C).
The external solution in this situation is hypertonic. Of course, in the situation in which, even in the presence of non-dispersible substances, there are no water flows, we would consider this an isotonic solution.
Therefore, while the osmolarity of a solution depends on the total concentration of diffusible and non-diffusible solutes and directly activates water flow, the tonicity is related to the concentration of non-diffusible solutes and the water flow is secondary to the flow of the solutes according to their concentration gradient.
Water transport. The transport of water, for example through the intestinal epithelium, occurs by osmosis, but is regulated by the mechanism outlined in Figure 4.8. The Na+/K+ ATPases present on the basal membranes forming the interstices between cells create a strong sodium gradient. This gradient increases from the interstitial fluid in the serosal zone of the intestinal epithelium towards the apex, near the intestinal lumen.
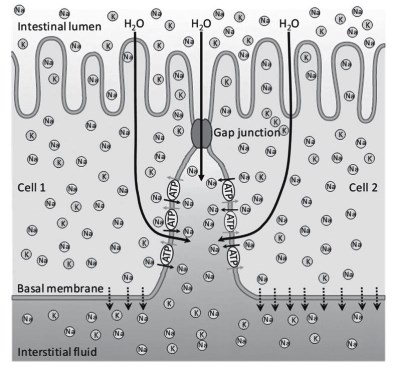
Figure 4.8. The passage of water (large black arrows) through the intestinal epithelium and gap junctions is regulated by the accumulation of sodium ions (Na) in the interstitium between cells due to the activity of a Na+/K+ ATPase (ATP) which exchanges sodium (small black arrows) and potassium (K and grey arrows) ions
The sodium gradient draws water by osmosis: water passes through both the apical membranes and the gap junctions between one cell and the next. The continuous passage of sodium through the epithelial cell therefore produces, by osmosis, a continuous passage of water in the same direction.
Water and sodium continuously diffuse through the basal membrane, which is not a barrier, to the blood capillaries. The water uptake model in Figure 4.8 is also known as the Curran and McIntosh model with three environments: the lumen, the interstitium and the serosal side of the epithelium.
It should be taken into account that whenever passage of sodium occurs, it is accompanied by the flow of chloride, which follows it passively to maintain the electroneutrality of the environment.
Water diffusion and flow occurring between two environments can be constant, establishing a dynamic equilibrium that is essentially stationary, coupled with the continuous cycling of the Na+/K+ ATPase. A different situation occurs for the absorption of sodium (Figure 4.12) and glucose (Figure 4.13) which occurs between the lumen and the blood vessels through the epithelial cells or, in some epithelia, the gap junctions (Data sheet 6.1).
In this case, there is a continuous supply of molecules, ions and water into the intestinal lumen as a result of digestion and into the lumen of the renal tubules as a result of ultrafiltration (Figure S4.2). Continuous passage of these substances through the epithelium are constantly moved into the bloodstream (Figures 4.8, 4.12, 4.13).
The draining action by the blood vessels, which keeps the concentration of sodium, glucose and water low in the deeper areas of the epithelium, allows a constantly high concentration gradient to be established from the intestinal lumen to the blood capillary. The continuous absorption of these molecules and ions produces a stable imbalance.
Ions, glucose and water absorbed by the intestinal epithelium must be transferred to tissue sites. Here, there is again a stable imbalance between the capillary and the cells that make up tissues. Cell metabolism drains nutrients continuously. The low cellular concentration of solutes induces a flow of substances from the capillaries to the tissues.
A third situation occurs when diffusion and flow are established between two environments in which fluid flows continuously, as happens, for example, with sea water and blood in the gills of fish (Figure 4.15).
Date added: 2024-07-10; views: 446;
