Donnan equilibrium (Gibbs-Donnan equilibrium)
The English chemist Frederick G. Donnan (Colombo 1870 - Canterbury 1956) demonstrated that in an experimental system consisting of two environments separated by a semi-permeable membrane, where the membrane is permeable to water and a salt but impermeable to a proteinate, the diffusible ions of the solute become unevenly distributed between the two compartments and generate, at equilibrium, a difference in potential at the sides of the membrane. This phenomenon is known as Donnan equilibrium, or Gibbs-Donnan equilibrium.
Let us assume an initial situation in which potassium chloride has been dissolved in room 1 at a certain concentration. The potassium chloride dissociates into potassium K+ ions and chloride Cl- ions (Figure S3.1A), which by diffusion, according to a gradient, pass from room 1 to room 2. At equilibrium, electro-neutrality will be restored and the ions will have
the same concentration in the two rooms (Figure S3.1B). At this point, a potassium salt of a non-diffusible A- anion, such as a protein, is added to environment 1 (Figure S3.1C), resulting in an increase in the concentration of the potassium ion in environment 1.
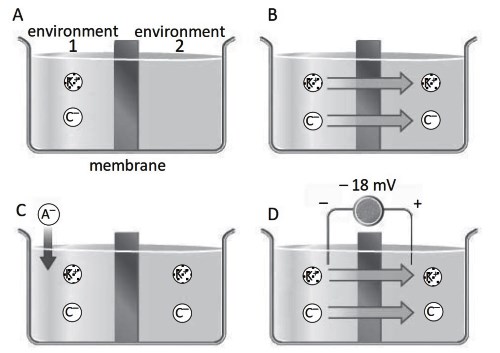
Figure S3.1. A salt dissolved in one environment (A) diffuses into the other environment (B) through the semi-permeable membrane and the concentrations become equal, but a non-diffusible anion (A–) placed in environment 1 (C) causes redistribution of diffusible ions (D)
The potassium ion tends to diffuse into environment 2 via a concentration gradient, also bringing along the chloride ion, the only anion that can move across the membrane. At equilibrium, the potassium ion is more concentrated in environment 1, where it has to neutralize the charges of the chloride ion and A-, than in environment 2, while the chloride ion is more concentrated in environment 2 (Figure S3.1D). The two environments again reach electroneutrality, i.e., the total number of positive charges is equal to the number of negative charges.
The equation can be proved
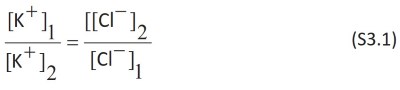
with unequal distribution of diffusible ions, characteristic of Donnan equilibrium.
The diagram in Figure S3.1C could represent the situation of a cell where environment 1 represents the inside and environment 2, the outside. The presence of the non-diffusible proteinate A- (Figure S3.1C) decreases the concentration of chloride and increases the concentration of potassium inside the cell compared to outside.
The more concentrated (by the same amount) chloride outside creates: a) a potential difference between inside and outside (Figure S3. 1D), b) a higher concentration of diffusible ions inside the cell, c) an increase in osmotic pressure with an inward flow of water that causes the cell to swell, a situation incompatible with its proper function, d) a certain hydrostatic pressure that opposes the osmotic pressure.
An equilibrium similar to Donnan's equilibrium, more correctly known as colloidosmotic pressure or oncotic pressure, is partly responsible for the osmotic pressure due to the presence of non-diffusible proteins. This equilibrium is established, for example, between the blood contained in the capillaries and the interstitial fluid, since the blood contains the plasma proteins to which the capillary membrane is impermeable, while the interstitial fluid is very poor in proteins.
More generally, the cell must be considered as a system similar to that represented by a Donnan equilibrium, but only as a simple introductory model for studying the mechanisms of ion distribution between two environments separated by a plasma membrane.
Compared with a functional situation such as that of the Donnan equilibrium, a) the cell maintains an almost constant volume; b) in the cell, active transport plays a decisive role in ion distribution; c) the plasma membrane is not perfectly semi-permeable; d) in reality, the cytoplasm is not a homogeneous aqueous solution; d) the activity of diffusible ions is generally much lower than their concentration because the salts are not completely dissociated.
Date added: 2024-07-10; views: 650;
