Ionic reserves. The membrane capacity
The average composition of the internal and external solution of a typical cell is given in Table 3.2. There are hydrophilic solutes including protein aggregates negatively charged at physiological pH, positive potassium, sodium, calcium and magnesium ions, anions such as chloride, carbonate and phosphate and hydrophobic molecules such as glucose and ATP.
The plasma membrane has a hydrophilic outer and inner layer, formed by the water-soluble polar heads of the phospholipids. In addition, there is a hydrophobic lipid bilayer in between, formed by the phospholipid tails.
Although hydrophilic molecules in aqueous solution can move freely, and therefore have a high probability of being close to the plasma membrane, they cannot cross the lipid barrier. On the other hand, hydrophobic neutral molecules are able to permeate into the lipid bilayer, but they need a "carrier" to move in the aqueous solution.
Because of its chemical and physical structure, the plasma membrane would therefore be an impermeable barrier to all types of molecules. However, the cell needs to exchange both hydrophilic and hydrophobic substances with the outside world. To do this, it can use specific integral membrane proteins for polar molecules or protein carriers for neutral compounds.
The chemical gradient. In order to perform a task, such as moving molecules from the inside to the outside of the cell, energy is required. If we look at Table 3.2, we see that the concentration of all the apolar molecules, amino acids, glucose and ATP, is different between inside and outside.
If, for example, the plasma membrane has integral proteins capable of passing glucose, the molecule is more likely to pass from the external environment, where the concentration is higher, to the internal environment, where the concentration is lower, because it is continuously consumed by the mitochondria to form ATP.
This transition occurs due to the presence of a concentration difference between the outside and inside: the chemical gradient, which provides the necessary energy. In cells, there is also a concentration difference for amino acids, proteins, ATP and other molecules. For each of these molecules, therefore, there is a chemical gradient independent of that of the others.
Looking again at Table 3.2, it can be seen that about 95% of the extracellular solution and about 75% of the intracellular solution consists of polar molecules, the ions derived from the dissolution of sodium, potassium, calcium, magnesium chlorides, various carbonates and phosphates in water. The two environments, like the whole organism, are electrically neutral. In fact, there are as many positively charged cations as there are negatively charged anions.
Looking at the table, it would seem that there is an excess of potassium ions in the intracellular solution compared to the anions. This is due to the fact that at the physiological pH of about 7.4, proteins are present in the form of protein anions with a high affinity for potassium, which can thus accumulate in the cell.
The membrane capacity. An additional fundamental role played by the lipid bilayers is charge buffer capacitance. Unbalanced charges (see for example Figure 3.15) inside or outside the biological membrane are immediately re-balanced by the membrane capacitor.
A transient capacitive current coordinates the accumulation of opposite charges across the membrane to re-establish electro-neutrality in the micro-environment formed by the membrane and the solutions in its immediate vicinity. This generates charge accumulation and thus the possibility to accumulate potential energy (Figure 3.17).
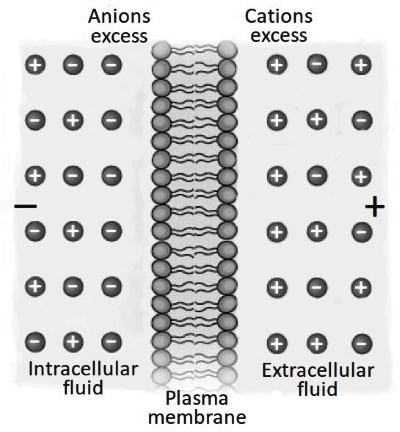
Figure 3.17. A model of a biological membrane capacitor. Phospholipid heads are the capacitor plates. Lipid tails work as a dielectric. Excess of cations or anions on one side of the membrane induces an equal number of counter-ions on the other side. This sets an electrically balanced system. However, in the close proximity of the membrane, there is a physical separation of charges, representing the ability of this structure to accumulate potential energy
There is a logic to follow for a clear understanding of the membrane capacitor structure/function relationship. The environment has an important role in shaping membrane structure. In terms of capacitor characteristics, an important element is the distance between the opposite polar heads (capacitor plates) created by the lipid tails (dielectric).
This is in the order of 8-10 nm and is a universal measurement for all biological cell membranes. This distance between the "plates" is instrumental to the forces of attraction for ions of opposite signs. With all of the parameters of Equation S2.3 constants, the only variable remaining is the cell surface. Thus, the time in which the membrane potential changes after stimulation (t, Equation S2.7) is directly proportional to the cell size.
Conductance or resistance. Ions immersed in a restricted environment move due to thermal agitation. For ions like Na, K and Cl, there is a high probability of colliding with the membrane. If the membrane contains integral proteins forming aqueous pores, it is possible for the charged particles to cross the membrane (Figure 3.18).
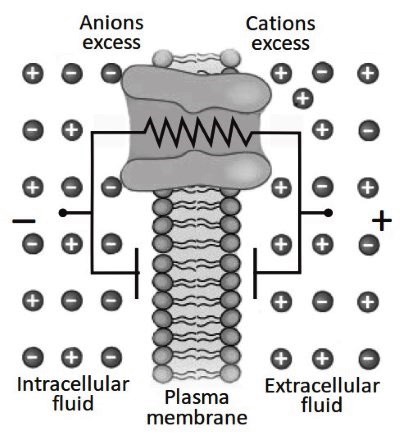
The parameters regulating ion movement through a channel protein are the resistance to passage (R) and the conductance (G) of the pore. These parameters being reciprocal (G = 1/R), the pore diameter is directly proportional to G and inversely proportional to R.
Similarly, but oppositely, the relationship is valid for channel length: this is directly proportional to R and inversely proportional to G. The two extremes for R are: (1) the membrane is sealed to ion movement, where the resistance is infinite and the conductance is zero and; (2) ionic permeabilities are fully open, where the resistance is the same as the physiological solution (100 Ω ∙ cm) and the conductance is maximum.
Date added: 2024-07-10; views: 410;
