Stress Sensing and Signal Transduction
The degree of molecular understanding of the different layers varies tremendously. Since the 1990s a lot of knowledge has been generated on the signal transduction cascades and transcriptional changes, but the stress-sensing mechanisms remain largely obscure. Generally accepted sensor proteins for temperature or hyperosmolarity have not been identified yet. Also, it is still difficult to specifically assign a molecular function to many of the putative protective proteins synthesised by cells during the course of a stress response.
A typical component of stress-activated signal transduction cascades is a transient increase in cytosolic Ca2+ concentrations. In fact, most interactions of plant cells with the abiotic or biotic environment involve Ca2+ signals (Dodd et al. 2010). This versatility may have had its origin in early constraints during evolution. Maintenance of cytosolic Ca2+ concentrations in the nanomolar range is mandatory to prevent precipitation of calcium phosphate, a salt with low solubility. Inorganic phosphate cannot be kept at such low concentrations, because of its integral role in energy metabolism (ATP).
The transporter- mediated export of Ca2+ from the cytosol out of the cell or into organelles generates an extremely steep electrochemical gradient, which can be exploited for very rapid signalling through transient Ca2+ influx into the cytosol. Specificity of the Ca2+ signal is achieved by spikes and oscillations, whose periods and amplitudes (the Ca2+ signature) are stimulus dependent (Fig. 2.16). Additionally, Ca2+ signals are perceived by large numbers of Ca2+ sensors such as calcium-dependent protein kinases (CDPKs), which provide further specificity.
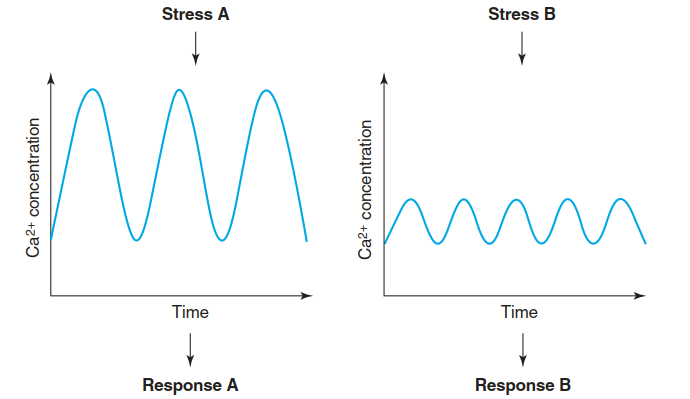
Fig. 2.16. Ca2+ signatures encode the specificity of stress responses. One of the earliest detectable events after perception of many different biotic and abiotic stress factors is a transient increase in cytosolic Ca2+ concentrations. In fact, the cytosolic Ca2+ concentrations oscillate. The specificity of the response is mediated by the amplitude and period of these oscillations, which are shaped by the specific activities and subcellular localisations of Ca2+ channels, Ca2+ pumps and Ca2+ sensor proteins. (Modified from McAinsh and Pittman
Another recurring scheme of stress signalling is the activation of phosphorylation cascades upon perception of a stimulus. Those most prominent in eukaryotes are mitogen-activated protein (MAP) kinase cascades—conserved modular signalling cassettes that regulate a wide range of stress responses and many other processes. The cassette consists of three principal components: a MAP kinase kinase kinase (MAPKKK), which activates a MAP kinase kinase (MAPKK), which activates a MAP kinase (MAPK). MAPKs then target a wide variety of proteins. Through phosphorylation they can modulate the activity or subcellular localisation of transcription factors, alter the activity of enzymes or trigger additional signalling cascades (Fig. 2.17).
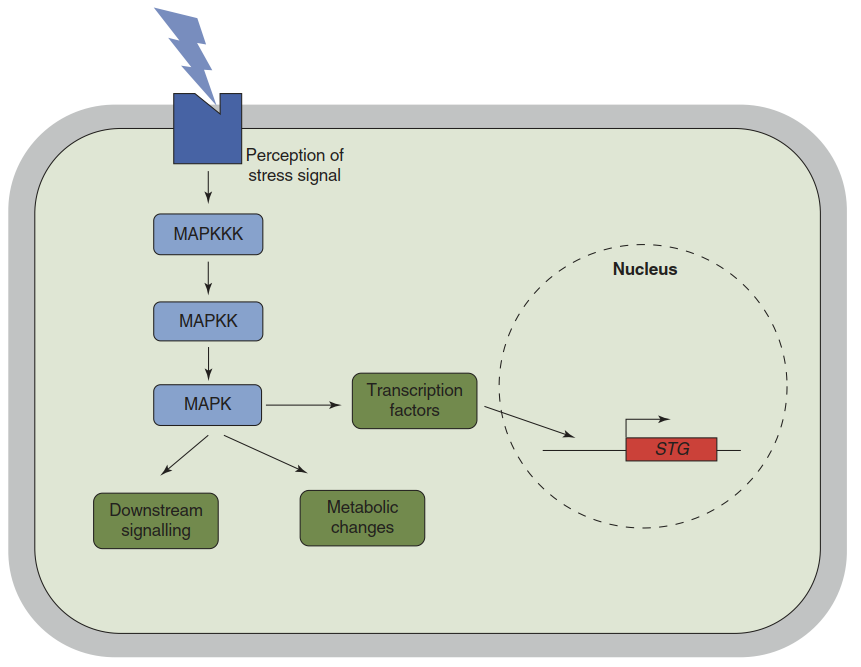
Fig. 2.17. Activation of stress acclimation by mitogen- activated protein (MAP) kinase pathways. Perception of a stress signal by a sensor or receptor protein is converted into the activation of a MAP kinase kinase kinase (MAPKKK). The MAPKKK activates a MAP kinase kinase (MAPKK) by phosphorylation; the MAPKK then activates a MAP kinase (MAPK). MAPKs have a variety of target proteins—for example, other signalling kinases, enzymes or transcription factors. The latter often move to the nucleus after phosphorylation and activate the transcription of stress tolerance genes (STG)
Plant genomes encode many different versions of these components. A. thaliana, for example, possesses about 60 MAPKKK genes, 10 MAPKK genes and 20 MAPK genes, theoretically enabling thousands of different pathways. The cascade is usually initiated when a signal such as a drop in temperature or the presence of a potential pathogen is perceived and a MAPKKK becomes phosphorylated. Several kinase cascades can be activated by one stimulus, and one kinase cascade can be activated by several stimuli. This provides a framework for the integration of different environmental cues via converging and diverging signalling pathways.
A third widely recruited element of signalling chains involved in stress acclimation, as well as in myriad developmental processes (e.g. the response to phytohormones such as auxin and gibberellic acid (GA)), is the control of protein stability. Many regulatory proteins are known that are not controlled, or are only weakly controlled, at the transcriptional level. Instead, their biological half-life is dependent on post-translationally added modifications that either flag the protein for degradation in the proteasome (poly-ubiquitination—the addition of several copies of the small protein ubiquitin) or inhibit such flagging (sumoylation—the addition of an ubiquitin-related protein, which prevents ubiquitination). The flagging is executed by so-called E3 ligase complexes, of which several hundred are encoded in a typical plant genome.
Date added: 2025-01-13; views: 343;
