Generation of Reactive Oxygen Species as a Consequence of Stress
ROS, resulting either from the organelle’s own metabolism or from import by diffusion, have been detected in all plant organelles that are surrounded by biomembranes. Organelles with high rates of electron flow, i.e., chloroplasts, peroxisomes/glyoxysomes and mitochondria—are major sites of ROS production. Because of their extreme reactivity, ROS are very toxic, causing peroxidation of lipids, oxidation of proteins and nucleic acids, enzyme inhibition or activation of programmed cell death (PCD).
The damage they cause depends on their concentrations and half-lives, their ability to diffuse through membranes and the activity of ROS scavengers in the various compartments of the cell (Scheibe and Beck 2011). ROS species carrying an unpaired electron, such as the oxygen radical anion (O2-) or the hydroxyl radical (OH·), cannot readily pass through cellular membranes, while singlet oxygen (1O2) and the relatively stable hydrogen peroxide (H2O2) are non-polar molecules and thus can permeate through biomembranes. Still, singlet oxygen has an extremely short lifetime and thus cannot migrate far from its site of origin.
The extreme reactivity of ROS requires high activity of ROS-scavenging reactions to maintain ROS and their toxic effects at a low level. Such a balance is impaired under stress. The classic case is the so-called Mehler reaction at the acceptor site of photosystem I (Fig. 2.21). When metabolic reoxidation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) is slower than its production and the ferredoxin pool thereby becomes overly reduced, the only amply available electron acceptor is the photo-synthetically produced oxygen, which can take up one electron, forming O2·-. Under normal conditions the rate of the Mehler reaction is low, as there is no enzyme catalysing the electron transfer to oxygen. However, metabolic reoxidation of NADPH can be limited by various abiotic stress conditions. One of them is low temperature.
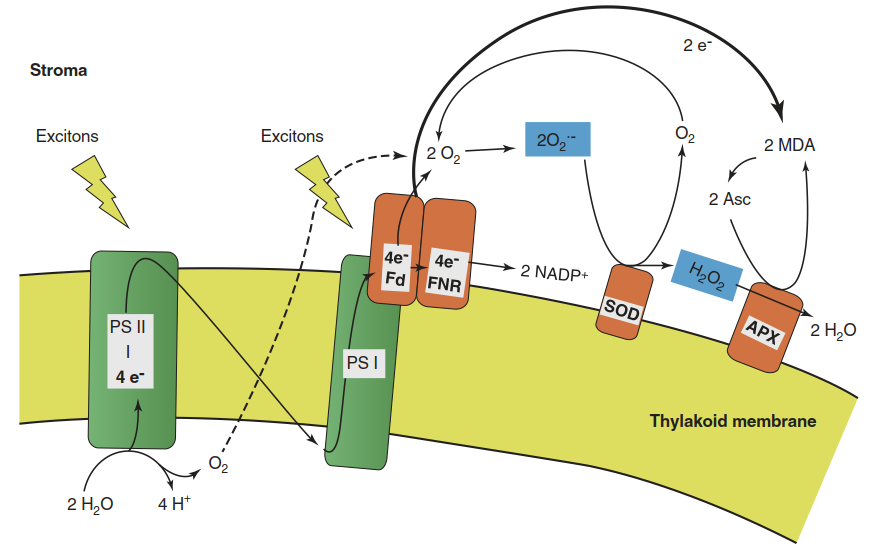
Fig. 2.21. Production and detoxification of O2·- and H2O2 in the photosynthetic electron flow. Transfer of an electron from ferredoxin (Fd) generates O2·-. Superoxide dismutase (SOD) catalyses the disproportionation of O2·-- to O2 and H2O2. H2O2 is reduced to H2O by ascorbate peroxidase (APX), yielding monodehydroascorbate (MDA). FNR ferredoxin-NADP+ reductase
The reactions of the photosynthetic electron flow from water to NADP+ are barely affected by low temperature (with a low Q10 value), while the rates of the enzymatic reactions in the Calvin cycle decrease considerably (with a high Q10 value). Thus, NADPH accumulates, causing an over-reduction of ferredoxin. A similar situation arises under drought. Stomata are closed and the CO2 concentration inside the leaves drops. Again, reoxidation of NADPH in the Calvin cycle is slowed down, this time because of a shortage of a reducible substrate.
Besides the Mehler reaction there are many other ways in which cellular homeostasis can be disturbed, inevitably causing an increase in the production of ROS. The resulting oxidative stress has been documented in plant cells exposed to heat stress, excessive UV radiation, ozone and other air pollutants, nutrient deficiencies or toxic minerals. Thus, elevated ROS levels are a common theme of stress conditions and can function as cellular indicators of stress (Mittler 2002).
A special form of ROS confined to the chloro- plast is singlet oxygen (1O2). Like the O2·- radical and H2O2 it is continuously produced by the plant’s metabolism, albeit at much higher rates during stress. Oxygen in the air possesses two unpaired electrons and thus occurs in the rather unreactive triplet state, 3O2. Energy transfer from excited chlorophyll to oxygen can cause spin reversal and formation of the far more reactive 1O2 — the “curse of the illuminated chloroplast” (Halliwell 2006). Because of its very labile paired electrons, 1O2 can easily react with many organic compounds, particularly with unsaturated molecules such as polyunsaturated lipids under formation of hydroperoxides. The rate of 1O2 formation increases strongly whenever excitation energy cannot be efficiently dissipated as photosynthesis, fluorescence or heat. This is the case, for example, under high-light conditions.
Date added: 2025-01-13; views: 371;
