Variations in Salt Tolerance: Plant Functional Types with Respect to Salinity
Plants vary greatly in their tolerance of salinity (Fig. 7.33). For example, after some time in a 200 mM NaCl solution, a salt-tolerant species such as sugar beet may have a reduction of only 20% in dry weight, a moderately tolerant species such as cotton might have a 60% reduction and a sensitive species such as soybean might be dead. A plant from a salt marsh (e.g. Suaeda maritima), however, may be growing at its optimum rate. Salt-sensitive species such as the monocot rice and the dicot A. thaliana are termed glycophytes, whereas species with a high salt tolerance or even salt requirement for growth are referred to as halophytes. While glycophytes are clearly more salt sensitive than halophytes, they still possess a basal tolerance mechanism, and most molecular knowledge on salt tolerance mechanisms originates from work with A. thaliana.
Not all developmental stages of a plant are equally sensitive to salinity. With respect to crops, this provides an opportunity to minimise salt injury at the sensitive stages by using irrigation water of differing salinity during the season. Notwithstanding their relatively high salt tolerance, sugar beet, barley and cotton are relatively sensitive during germination or early seedling growth. In contrast, corn, pea and beans are more sensitive during later stages of development. In tomato, salt tolerance is low in young plants, becomes much higher during vegetative growth and decreases again during flowering.
Salt Tolerance Mechanisms. At the cellular level, plants have several principal mechanisms to cope with salinity (Fig. 7.29). They can:
- Minimise initial entry into the root
- Maximise efflux from the root into the soil
- Minimise loading into the xylem or maximise retrieval from the xylem fluid before Na+ reaches the shoot
- Maximise recirculation out of the shoot into the phloem
- Maximise intracellular compartmentation or allocation to particular parts of the shoot (e.g. pith cells or old leaves)
- Secrete salt via glands to the surface of the leaf or into specific bladder hairs
The extent to which these mechanisms operate in plants varies from species to species, and even within species, and depends on the severity of the stress. Because the control—especially of Na+ influx into the root and efflux from the vascular parenchyma into the xylem apoplast—is weak owing to the limited specificity of many nutrient transporters, the most important cellular aspects of salt stress tolerance are efficient removal of Na+ from the cytosol especially in young tissues and regulation of Na+ distribution within the plant.
Maintenance of low cytosolic Na+ is achieved by secretion into the apoplast or sequestration in the vacuole. This applies to cells in the outer part of the root—that is, the rhizodermis and the cortex—as well as the metabolically active cells in the shoot. Fig. 7.34 shows the basic mechanisms by which the cell can manage its cytosolic Na+ concentration at the expense of ATP.

Fig. 7.34. Cellular Na+ tolerance mechanisms
The dominant systems in the plasma membrane, as well as in the tonoplast, are Na+/H+ antiporters, which use the proton gradients produced by H+- ATPases or the vacuolar pyrophosphate-driven proton pump to extrude Na+ from the cytosol. The transporters were first identified in A. thaliana and named SOS1 (salt overly sensitive, the protein in the plasma membrane) and NHX (for Na+/H+ exchanger). Their activity is an important component of Na+ tolerance in both glycophytes and halophytes. Knockout or knock-down of the antiporter expression results in a dramatic increase in salt sensitivity in A. thaliana, tomato and also the halophyte Eutrema salsugineum (formerly known as Thellungiella salsuginea) (Fig. 7.35).
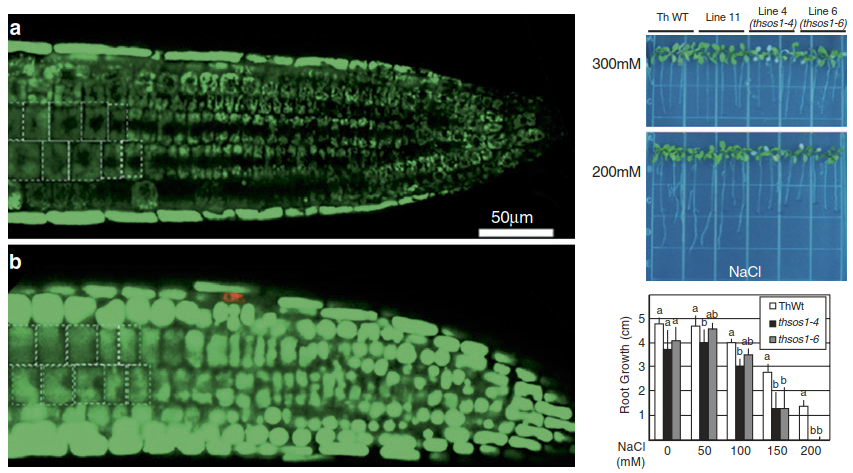
Fig. 7.35. Knock-down of SOS1 in the halophyte Eutrema salsugineum formerly Thellungiella salsuginea causes stronger Na+ accumulation in root cells and compromises Na+ tolerance. Left: Accumulation of Na+ in root cells visualised with a fluorescent dye (a wild type, b SOS1 RNA interference (RNAi) plant). Right: SOS1 RNAi plants (thsos1 lines) are more NaCl sensitive than wild-type plants
NHX antiporters belong to the large cation/ proton antiporter 1 (CPA1) family which, by sequence similarity and intracellular localisation, is further subdivided into vacuolar (class I) and endosomal (class II) NHX transporters. Most of the plant species sequenced to date contain both types of NHX. The cation selectivity of AtNHX1 represents an instructive example of post-translational transporter modification (Fig. 7.16). It appears to be controlled by the C-terminal domain reaching into the lumen of the vacuole. Depending on the vacuolar Ca2+ concentration and the pH, it binds to a calmodulinlike protein, AtCaM15. Interaction with AtCaM15 decreases the Na+ transport activity of AtNHX1 while maintaining the K+ transport activity almost unchanged (Yamaguchi et al. 2013). Under normal physiological conditions—that is, a high vacuolar Ca2+ concentration and an acidic pH— binding of AtCaM15 favours the K+/H+ antiport mode. However, as salinity stress causes vacuolar alkalinisation, AtCaM15 dissociates from AtNHX1, which then exhibits higher Na+/H+ antiport activity and promotes sequestration of Na+ into the vacuole. Overexpression of NHX genes improves the salt tolerance of a range of plant species, with a concomitant increase in tissue Na+.
Vacuolar sequestration and efflux into the apoplast are also the cellular mechanisms underlying salt secretion in specialised leaf structures. In salt bladders (specialised trichomes), salt accumulates in the large central vacuole of the bladder cells. Bursting of the cells then deposits salt on the leaf surface. In salt glands (Fig. 7.36), vacuolar vesicles filled with salt fuse with the plasma membrane for exocytotic release of salt, or Na+ ions are directly transported out of the cell across the plasma membrane. Thus, it can be postulated that NHX- and SOS1-like proteins play a key role also in these specific adaptations.
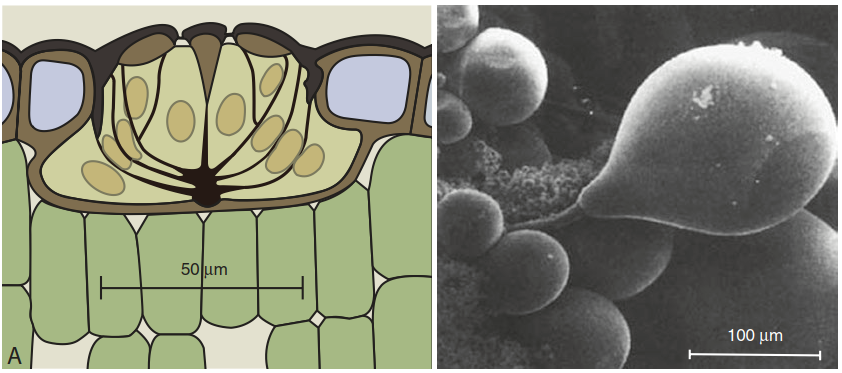
Fig. 7.36. Mechanisms of salt secretion: longitudinal section of a salt gland in a sea lavender (Limonium vulgare) leaf and salt hairs as protuberances of the leaf epidermis in Atriplex hymenelytra
Another major mechanism of salt tolerance is the control of Na+ translocation to the shoot. This is largely a function of re-uptake of Na+ from the xylem into xylem parenchyma cells. The transporters responsible for this retrieval are those in the HKT (high-affinity K+ transporter) family. HKTs belong to a superfamily of potassium transporters, which have been found in microorganisms, yeasts, plant cells and parasites such as trypanosomes.
Two classes can be differentiated on the basis of functional and structural traits: class I, which is more selective for Na+; and class II, encompassing K+/Na+ co-transporters. A. thaliana AtHKT1;1 loss-of-function mutants are Na+ hypersensitive and accumulate more Na+ in the leaves. Conversely, overexpression under the control of a stele-specific promoter has been shown to reduce Na+ transport to the leaves and result in higher salt tolerance (Mpller et al. 2009). AtHKT1;1 resides in the plasma membrane of xylem parenchymal cells and phloem tissues. The latter explains recirculation of Na+ from the shoot to the root via AtHKT1;1, which may contribute to salt tolerance.
A common acclimative response of a plant to salinity is lowering of the water potential of its cells. This is achieved by accumulation of low molecular weight compounds (the so-called compatible solutes or osmolytes) in the cell— for example, quaternary ammonium compounds such as glycine betaine, polyamines, open-chain sugar alcohols (polyols) such as mannitol and glycerol, oligosaccharides such as trehalose, and proline. In addition to their colligative effects, osmolytes can partially replace water, thereby stabilising proteins and cellular substructures. Some osmolytes are rather salinity spe- cific—that is, they are less commonly produced under other stresses such as drought. Very common osmolytes in halophytes—for example, mangroves or ice plants—are cyclic sugar alcohols or cyclitols.
In contrast to the open-chain polyols, they show slow metabolism, which prevents their consumption in situations of throttled carbohydrate availability (e.g. when stomata are closed), thus safeguarding the osmolyte function. Their biosynthesis starts from glucose-6-phosphate, which is cyclised to inositol-3-P, the basic compound for a variety of cyclitols. They are varied by relatively simple biochemical reactions such as epimerisation (e.g. L-quebrachitol) or transfer of methyl groups (e.g. D-ononitol), which renders them metabolically rather inactive. Because halophytes can tolerate comparatively higher cytosolic salt concentrations owing to compatible solute accumulation and efficient vacuolar sequestration, they can also use Na+ and Cl- as osmolytes to lower the osmotic potential.
Like cells affected by low water availability, salt-stressed cells synthesise proteins that are assumed to protect cellular structures such as membranes and protein complexes by associating with them.
Date added: 2025-02-01; views: 361;
