Metal Hyperaccumulators as Models for Adaptation to Extreme Environments
Other essential micronutrients such as Zn or Ni can reach toxic concentrations in some soils too. While toxic Zn concentrations are largely restricted to mining-impacted soils, potential Ni toxicity of serpentine soils is more common. A number of plant species have evolved the ability to colonise metal-rich sites. They are called metallophytes.
As described in this chapter, the concentrations of metals in plant tissues are under tight physiological control. Many elements are toxic when present in excess. However, approximately 500 taxa (i.e. about 0.2% of all flowering plant species) have evolved the ability to hyperaccumulate metals or metalloids (arsenic, selenium) in their leaves. Hyperaccumulation is defined as a metal concentration that is above an element-specific threshold in above-ground tissues of plants grown in the field. This threshold should be a concentration that is two to three orders of magnitude higher than what is normally found in plants growing in soils that are not enriched with particular metals, and one to two orders of magnitude higher than what non-hyperaccumulating species show at a site where the hyperaccumulator grows (Kramer 2010).
For example, the hyperaccumulation thresholds are 100 parts per million (ppm) for Cd, 1000 ppm for Ni and As, and 3000 ppm for Zn, while plants normally contain, for example, about 50-100 ppm Zn in their organs. Important aspects of this definition are (a) that extreme accumulation is found in plants grown in the natural habitat (not only in plants cultivated under laboratory conditions) and (b) that the accumulation in above-ground tissues is due to active translocation via the roots and not caused by aerial deposition onto the leaves.
Hyperaccumulators represent a subgroup of the metallophytes—that is, plants that are part of a special type of vegetation found on metal-rich sites. Such sites can be rich in metals either naturally (geogenic) or because of human activities such as metal mining or processing (anthropogenic). Typical geogenically metal-rich habitats are serpentine (“ultramafic”) soils with high concentrations of Ni; calamine soils rich in Zn; soils in the African copper belts characterised by high concentrations of Co, Cu, Cr, Ni and Zn; and seleniferous soils enriched in Se (Baker 1989). An example of typical metallophyte vegetation is the “Galmei flora” found in calamine soils in Belgium and the region around Aachen in Germany. A characteristic species is the endemic Viola calaminaria.
All metallophytes have evolved mechanisms to survive and reproduce on metal-rich soil. They share the ability to tolerate metal or metalloid concentrations that would strongly inhibit or even kill most other plants. With respect to the accumulation of metals, three strategies are distinguished. Many metallophytes are able to grow in the presence of metal/metalloid concentrations that are intolerable for most plants, because they can efficiently exclude the metal from their cells. Even in the presence of high concentrations of bioavailable metal in the soil, the concentration within the plant is maintained at a very low level (Fig. 7.27). Examples are the aforementioned V. calaminaria and other more common metallophytes such as Armeria maritima and Silene vulgaris.
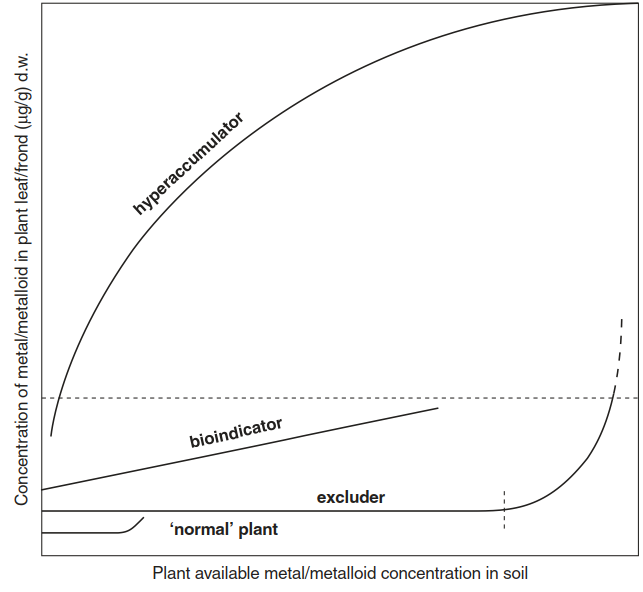
Fig. 7.27. Metal accumulation behaviour of metallophytes and of plants not adapted to metal-rich sites
Some metallophytes are bioindicators. They can tolerate a wider range of concentrations than normal plants, and the accumulation in leaves is linearly correlated with concentrations of available metals in the soil. The third strategy is hyperaccumulation. The plants very efficiently translocate metals from the soil solution via root uptake and long-distance transport into the shoots. Some hyperaccumulator species even hyperaccumulate when growing on sites not enriched in metals at all. They are pseudo- metallophytes, meaning that they occur not only at metal-rich sites but also at sites with normal metal availability in the soil. Examples of such species are the model hyperaccumulators Arabidopsis halleri and Noccaea caerulescens (in the Brassicaceae; see below).
A given metallophyte species does not tolerate toxic concentrations of any metal. Instead, naturally evolved hypertolerance—that is, a degree of metal tolerance exceeding the tolerance found in most plant species—is specific to certain metals. Plants adapted to serpentine soils can thrive in the presence of high Ni concentrations but not necessarily in the presence of high Zn concentrations. There is pronounced variation even within species. Accessions of N. caerulescens adapted to calamine soils tolerate Zn and Cd very well but are sensitive to Ni. Conversely, accessions from ultramafic sites are extremely Ni tolerant yet are as sensitive to Zn and Cd as non-metallophytes (Halimaa et al. 2014).
The large majority of hyperaccumulator species—that is, about 450 out of 500—hyperaccumulate nickel and typically occur in serpentine (ultramafic) soil. Hotspots for Ni hyperaccumulators are Cuba and New Caledonia. They are rich in serpentine sites, and more than 150 Ni-hyperaccumulating species grow on these islands. Around 15 taxa hyperaccumulate either Zn, As or Se. A few species accumulate Cd (Kramer 2010). Hyperaccumulating species are strongly overrepresented among the Brassicaceae, indicating a propensity of species in this family to evolve hyperaccumulation.
The adaptation of metallophytes to metal-rich soils has attracted attention from evolutionary biologists and ecologists because the toxicity of metals exerts extreme selective pressure. On serpentine soil a large number of endemic species are found, indicating the need for specific adaptations to cope with such edaphic conditions (which include a high Mg to Ca ratio and scarcity of N, P and K, in addition to high metal concentrations). Colonisation of sites that have been metal contaminated by human activities represents an example of rapid evolution—of “evolution in action”, as stated by Antonovics et al. (1971). Within a range of a few metres the environment can differ dramatically, allowing many plants to grow in a meadow and allowing only very few adapted plants to establish themselves on a neighbouring heap of mining waste.
Since the 1990s, research into the ecophysiology and genetics of metallophytes has, in addition, been fuelled by several applications envisioned for metal-hypertolerant plants. They can be used for phytoremediation of metal-contaminated areas—for example, mining sites— to enable gradual revegetation. Metallophytes can facilitate the growth of other plants by reducing the bioavailability of toxic pollutants (phytostabilisation). Metal-hyperaccumulating plants could even be used for phytoextraction to remove metal contaminants (Salt et al. 1998). A related application is phytomining to exploit substrates that are too poor for conventional mining.
Mechanistic insights into the evolution of hyperaccumulation can illustrate the path leading to adaptation to extreme environments or, more generally speaking, the emergence of new traits in nature. Metal hyperaccumulators show exceptional mobility of metals and metalloids, which enables the transfer to above-ground tissues and thereby a fundamentally different partitioning of metals between below-ground and above-ground tissues. The relatedness of hyperaccumulator species such as N. caerulescens and A. halleri to the molecular genetics model A. thaliana has enabled molecular approaches to the unravelling of metal hyperaccumulation. Intra- and interspecific crosses—for example, between contrasting N. caerulescens accessions or between A. halleri and its non-hyperaccumulating relative Arabidopsis lyrata—have revealed the genetic architecture of metal hypertolerance and metal hyperaccumulation.
The two traits are partially independent. Some of the mapped quantitative trait loci are important for both characteristics, others only for one of the two. Several studies compared the transcriptomes of hyperaccumulators with that of A. thaliana (Becher et al. 2004; Weber et al. 2004). The fundamental realisation was that many genes involved in metal homeostasis in normal plants show altered regulation in hyperaccumulators. Genes encoding metal transporters and enzymes catalysing the synthesis of metal ligands are constitutively more strongly expressed (Fig. 7.28).
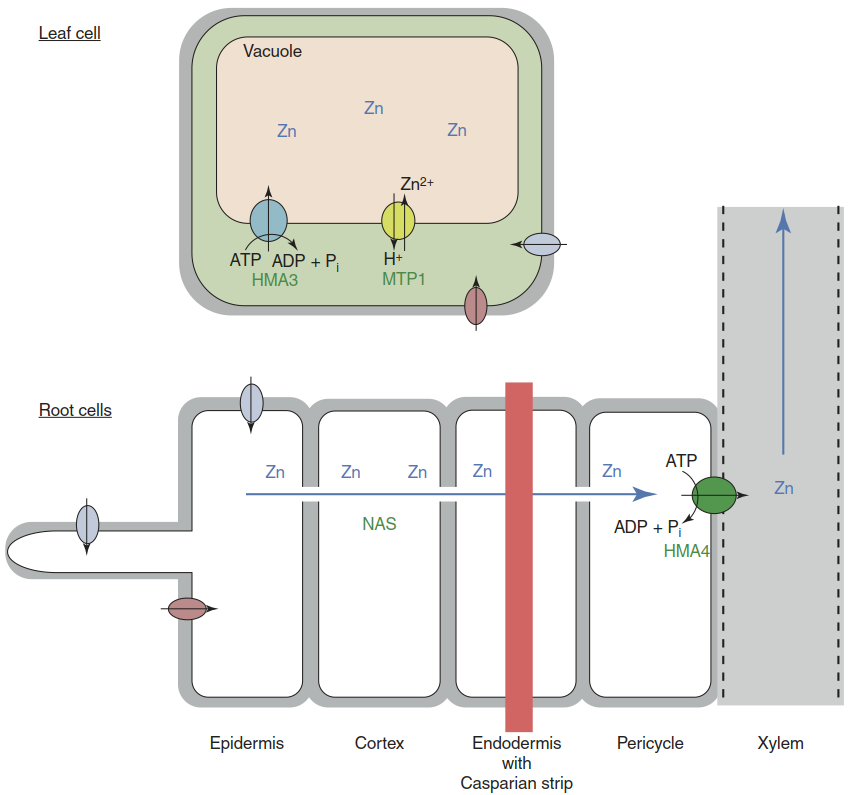
Fig. 7.28. The current model of Zn hyperaccumulation. In Zn-hyperaccumulating species such as Arabidopsis halleri and Noccaea caerulescens, Zn mobility in the symplast is higher because of greater nicotianamine synthesis by nicotianamine synthases (NAS), more Zn is pumped into the xylem (metal pump HMA4) and more Zn can be transported into the vacuoles of leaf cells (MTP1 and HMA3). The corresponding genes are all more strongly expressed in hyperaccumulators because of copy number expansion and cis-regulatory changes. Indicated uptake transporters are molecularly unidentified
The best-documented example is the metal-pumping ATPase HMA4, a protein that effluxes Zn ions across the plasma membrane (Sect. 7.3.4). In A. thaliana, HMA4 loads Zn into the xylem for translocation to the leaves. Much stronger expression of HMA4 in A. halleri results in very efficient root-to-shoot translocation of both Zn and Cd. In addition the efflux activity protects root cells from Zn and Cd toxicity (Hanikenne et al. 2008). This difference explains a substantial fraction of the Cd/Zn hypertolerance and hyperaccumulation of A. halleri. Higher expression evolved through an expansion of the gene copy number and variation in promoter sequences. The proteins apparently do not differ in affinities, activities or any other functional features.
Thus, an important conclusion of general importance is that adaptation has evolved through transcriptional changes via copy number variation and changes in cis-regulatory sequences— that is, rather subtle changes that can easily occur and then be selected. Indeed, for the triplicated HMA4 locus in the A. halleri genome, strong signs of a selective sweep were detected (Hanikenne et al. 2013). Among individuals from different populations, nucleotide diversity was strongly reduced around the HMA4 locus. This is indicative of strong selection causing rapid spread of this particular allele in A. halleri populations.
Sodium Toxicity
The soils of more than 6% of the world’s terrestrial surface contain high concentrations of salt, mostly NaCl. To a lesser extent, Na-carbonate and Ca-sulphate are also found, especially in the vicinity of salt lakes. Needless to say, two thirds of the Earth’s surface—namely, the oceans—rep- resent saline habitats. Halogenides of alkali and earth alkaline ions are easily soluble in water and thus these ions are washed out from suboceanic or terrestrial minerals of the Earth’s mantle and finally accumulate in the sea.
From there, saline aerosols are transported landwards by the wind, leading to continuous salt deposition not only in the coastal regions but also further inland. In arid and semi-arid areas, upward movement of the soil water results in deposition of dissolved salt upon evaporation of the solvent, frequently giving rise to salt crusts. This process also takes place in irrigated arid areas. It is estimated that by the middle of the current century, increased salinisation will result in up to 50% arable land loss (Wang et al. 2003). The impact of soil salinity on agriculture is enormous, as it affects plants during their entire life cycle and results in huge losses in biomass production and yields.
Soils are considered saline when the electrical conductance exceeds 0.4 Siemens per metre. This value corresponds to approximately 40 mM NaCl and an osmotic potential of -0.2 MPa. The threshold value is derived from agriculture. Many crops react with yield reduction when grown in soils of higher salinity. Salt tolerance is usually determined as the percentage biomass production or crop yield in saline versus control conditions over an extended period of time, or in terms of survival, which is especially useful in experiments with seedlings.
The conductivity of seawater (3% salt: 480 mM Na+, 50 mM Mg2+ and 560 mM Cl-) is 4.4 S/m— more than ten times the threshold for soil salinity—with an osmotic potential of -2.7 MPa. The conductivity of water used for irrigation must be less than 0.2 S/m, notwithstanding the fact that some plants—for example, glassworts (Salicornia species) or even special cultivars of barley (cv. California Mariout)—require or at least tolerate irrigation with seawater. For as yet unknown reasons, salinity in soils is often accompanied by toxic concentrations of boric acid.
Na+ leaks into plant cells via Ca2+-permeable non-selective cation channels (NSCCs) or K+/ Na+ transporters. The molecular identities of these proteins are still uncertain. Many K+ transport systems such as HAK/KUP family members have some affinity for Na+. High- affinity Na+ uptake has also been observed but is unlikely to play a role under conditions of salt stress with high external Na+ concentrations (Munns and Tester 2008). The Na+ electrochemical potential gradient across the plasma membrane suggests that facilitated diffusion is the principal mode of Na+ influx, while Cl- is transported against the electrochemical potential.
Uptake therefore requires proton coupling, while efflux can be passive. Anion influx can be passive too, provided that a permeable anion channel is present and the concentration gradient of the ion across the membrane is high enough, as can be the case in saline environments. Furthermore, depolarisation will result from the uptake of cations such as Na+. This lowers the electrochemical potential gradient and facilitates Cl- uptake. Thus, exposure of a cell or tissue to high salt concentrations results primarily in passive influx of Cl-, followed by active uptake after the membrane potential has returned to more negative values.
Salt stresses plants in several ways (Fig. 7.29): by dehydration, Na+ (and Cl-) toxicity, nutrient imbalances and reactive oxygen species production (ROS production). Saline soil solutions have a very negative osmotic potential, which has to be overcome by the water-absorbing surface of the plant, typically the root.
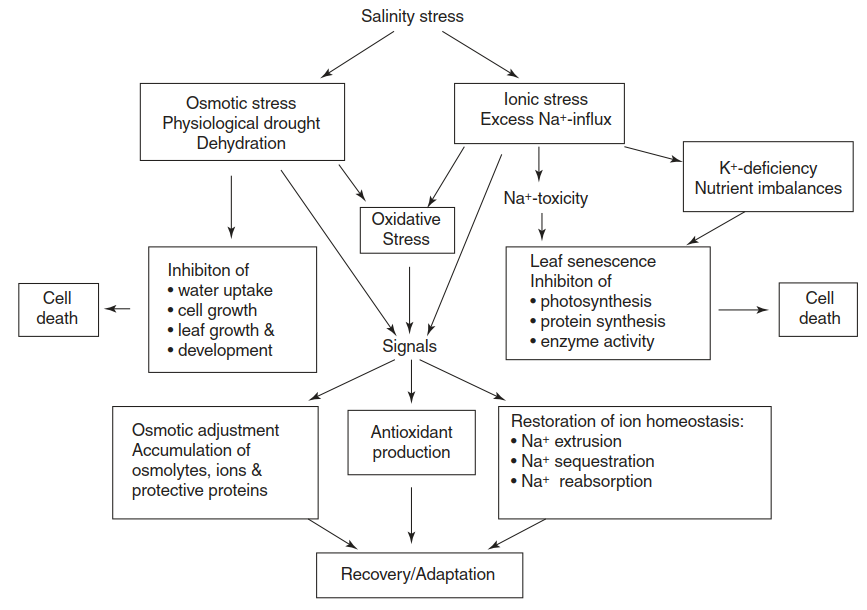
Fig. 7.29. Components and physiological consequences of salinity stress for plants, and mechanisms of adaptation conferring salt tolerance
Shortage of water affects growth of cells and plant organs. Thus, for water uptake, the root cells have to produce and maintain an even more negative osmotic potential. Na+ in particular, but also Cl-, can be regarded as biologically aggressive solutes on account of their small ionic diameters and the corresponding high surface charge.
These properties result in high attraction for water molecules and thus the binding of much water in water shells, which enhances intracellular water scarcity. Also, accumulation of small cations such as Na+ can strongly interfere with the intracellular balance of ion pools and charges, affecting the membrane potential, which may result in inactivation of voltage- dependent membrane functions. This is especially the case when K+ is displaced by Na+ with its higher charge density. Other inorganic plant nutrient relations—for example, of Ca2+, Mg2+ and anions such as nitrate and malate—may be affected as well.
Date added: 2025-02-01; views: 410;
