Pathogenicity Mechanisms
Pathogens have to gain access to the plant’s resources and be able to grow and reproduce rapidly within plant tissues. Both processes require effective suppression of the plant’s immune system. Invasion of plants is achieved through widely different strategies depending on the type of pathogen. As mentioned, viruses are transmitted by insect vectors. The same applies to some phytopathogenic bacteria such as phytoplasma. Most bacteria do not have the ability to actively penetrate plant surfaces.
However, after entry through wounds or stomata into the apoplast they can degrade plant cell walls via the secretion of a suite of enzymes such as cellulases and pectin- ases. Cell wall degradation causes disease symptoms such as softening of tissues. For some Erwinia species it is known that the bacteria “wait” to secrete these enzymes only when a sufficiently large number of bacterial cells are present at the infection site to overwhelm the plant. Earlier release would only alert the plant immune system. The “waiting” is mediated by quorum-sensing mechanisms, which bacteria use to monitor the density of a population.
Two other elements of bacterial virulence are common to both plant and animal patho- gens—namely, toxins and effectors. The latter are molecules that bacteria transfer into host cells via secretion systems (e.g. type III secretion system; Fig. 8.2). Effectors influence host cells through specific interaction with target structures. They interfere with the plant immune system and render the plant environment more favourable for the bacteria. An example is the protein avrBs3 secreted by the tomato pathogen Xanthomonas campestris. Even though it is encoded by the bacterial genome, it has the features of a eukaryotic transcription factor. After release into the plant cell it enters the nucleus and interacts with specific promoter elements to activate a group of genes that collectively cause hypertrophy of cells and thereby disease (Kay and Bonas 2009) (Fig. 8.2). A particularly famous example of an effector molecule is the transfer DNA (T-DNA) of Agrobacterium tumefaciens that triggers tumour growth and the synthesis of organic nutrients for the bacteria.
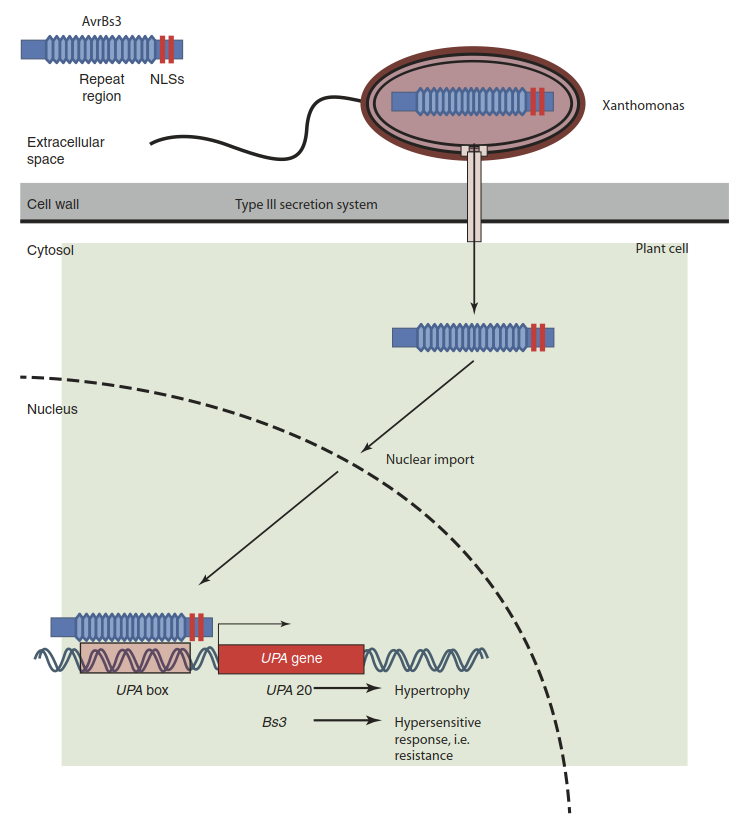
Fig. 8.2. Interaction between the avirulence gene avrBs3 and the resistance gene Bs3. The bacterial pathogen Xanthomonas attacks tomato and pepper cells by injecting effector proteins through the type III secretion system. One of the effectors is avrBs3. It has features of a eukaryotic transcription factor—for example, nuclear localisation signals (NLSs). After entry into the nucleus it activates genes (UPA genes, upregulated by AvrBs3) whose activity causes disease. The matching tomato resistance gene, Bs3, which is present in some tomato cultivars, carries similar promoter elements (UPA box). However, Bs3 activation triggers a cell death programme. The cell undergoes the hypersensitive response, and Xanthomonas cannot spread further
Like many effectors, toxins appear to often function as suppressors of plant defences. This is illustrated by coronatine, a molecule synthesised by Pseudomonas syringae. Coronatine mimics the phytohormone jasmonate and interacts with the jasmonate receptor COI1. Activation of the jasmonate pathway reverses the closure of stomata, which is an integral part of the plant’s defence response.
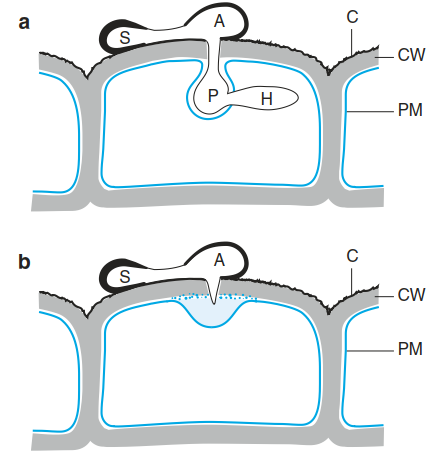
Fig. 8.4. Compatible a and incompatible b interactions during infection by a fungus. a A germinating spore (S) develops a germ tube from which an appressorium (A) is formed. Mechanical pressure building up in the appressorium, together with the secretion of cell wall-degrading enzymes, allows entry of a primary hypha (P) through the cuticle (C) and cell wall (CW). Later, secondary hyphae (H) grow into the host cell. b Defence against a fungal infection in a resistant plant. Pathogen recognition triggers rapid wall thickening under the appressorium. As a result the fungus is unable to penetrate the outer wall of the epidermal cell
Although fungi and oomycetes are unrelated, their infection strategies and growth forms as plant pathogens share several similarities, so they can be described together. An infection starts from a spore adhering to the surface of a plant and the germination of this spore. Biotrophic pathogens form an appressorium (Fig. 8.4). Within this appressorium, pressure builds up, exerting a mechanical force that, together with the activity of secreted enzymes (cutinases, cellulases and pectinases), disrupts the plant cuticle and cell wall. A feeding structure called a haustorium then develops behind an invagination of the plant cell plasma membrane (Fig. 8.4). The plant cell is kept alive but now supplies organic and inorganic nutrients to the invading fungus.
Necrotrophic fungi do not build haustoria. Instead they overpower a plant with non-specific or host-specific toxins and with cell wall-degrading enzymes. The plant cells die and the fungi utilize the dead organic material. An intensively studied potent toxin is fusicoccin, a diterpene synthesised by Fusarium species. It causes rapid wilting of exposed plants by constitutively activating the plasma membrane H+-ATPase via the modulation of 14-3-3 proteins. In guard cells this activation prevents the closure of stomata because the cells remain hyperpolarised.
Like bacteria, fungi and oomycetes produce effectors to suppress the plant defence response and to disturb plant development or metabolism. The phytohormone gibberellin was discovered as a molecule the fungus Gibberella fujikuroi uses to trick rice seedlings into abnormal growth. This increases their risk of lodging and thereby falling victim to the fungus. Ustilago maydis, a biotrophic pathogen of maize (causing maize smut, characterised by large tumours), secretes more than 100 effector proteins during its attack. One of them is the enzyme chorismate mutase. Following uptake into plant cells it modulates the phenylpropanoid pathway in a way that synthesis of the defence hormone salicylic acid is disturbed.
Nematodes penetrate plant cell walls with feeding stylets. Movement through root tissue is aided by the release of cell wall-degrading enzymes (Davis and Mitchum 2005). Inside plant cells, feeding tubes are formed that extract nutrients from the host. Molecules secreted by the nematode trigger dramatic changes in the regulation and metabolism of root cells, which eventually give rise to giant cells or symplastically connected cells that form syncytial feeding structures for the nematode.
Date added: 2025-02-01; views: 373;
