Koch's Postulates Revised: Aeromonas and Aerolysin in Ulcerative Colitis Pathogenesis. Science Insight
Introduction to Microbial Pathogenesis and IBD. Koch's postulates, formulated by Robert Koch and Friedrich Loeffler in 1884, established a foundational framework for identifying microbial pathogens. These criteria require that the microorganism is found in all disease cases, can be isolated and cultured, causes disease in a healthy host, and can be reisolated. These principles have been revised to account for unculturable microbes, like viruses, and to recognize microbial genes as disease agents. Despite these revisions, no microorganism fulfilling Koch's revised postulates has been definitively identified as the cause of ulcerative colitis (UC). In a pivotal study, Jiang et al. report that a specific bacterial strain, producing the toxin aerolysin, drives gut inflammation in mice, suggesting a novel mechanism for UC progression.
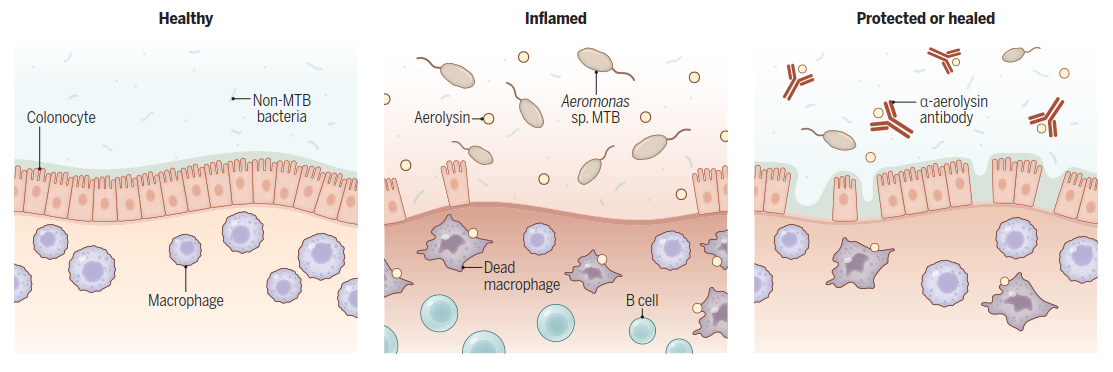
Aerolysin makes the colon susceptible to inflammation. Aeromonas sp. macrophage-toxic bacteria (MTB) isolated from patients with ulcerative colitis produce aerolysin, which is toxic to gut macrophages. Loss of macrophages leads to inflammation and accumulation of B cells and other components of the immune system, which weakens the colon's defenses. Anti-aerolysin ( -aerolysin) antibodies restore the gut mucosa to a healthy state.
Understanding Inflammatory Bowel Disease (IBD). Inflammatory bowel disease (IBD) is an umbrella term for chronic conditions where the immune system attacks the digestive tract, causing inflammation, abdominal pain, diarrhea, and fatigue. The most common forms are Crohn's disease and ulcerative colitis. UC specifically inflames the colon and rectum lining, leading to ulcers and bleeding. Early bacteriological cultures from UC patients failed to pinpoint a specific causative agent, unlike successes with pathogens like Salmonella. Genomic sequencing, while unsuccessful in finding a singular pathogen, revealed altered gut microbiota composition in patients. Epidemiological studies linking UC prevalence to urbanization and modern lifestyles further support complex environmental causes.
Historical Context and the Microbiota in Ulcerative Colitis. The search for an infectious cause of UC has been challenging. Traditional cultivation methods did not yield a consistently associated microbe. Advanced genomic analyses confirmed dysbiosis—a significant imbalance in the gut microbial community—in UC patients, but no single pathogen emerged. This absence of a clear etiological agent prompted researchers to explore alternative mechanisms, such as toxin-mediated damage from commensal bacteria. The study by Jiang et al. shifts focus from a primary infectious cause to a pathobiont—a potentially harmful resident bacterium—that exacerbates disease under permissive conditions.
Jiang et al.'s Methodology: Linking Immunology and Microbiology. Jiang et al. analyzed colon biopsies from UC patients using histology and immune cell profiling. They discovered a near absence of tissue-resident macrophages in the subepithelial mucosa, a layer crucial for immune surveillance. Complementary experiments in mice showed that chemical or genetic depletion of these gut-resident macrophages made the colon vulnerable to chemically induced inflammation. This pointed to a critical protective role for these cells and suggested their loss might be a key event in UC pathogenesis, potentially triggered by a microbial product.
Hypothesis: A Bacterial Toxin Targets Immune Cells. The researchers hypothesized that a microbiota-derived agent depletes macrophages in UC. Screening patient fecal samples, they identified aerolysin, a pore-forming toxin secreted by bacteria of the Aeromonas genus. In vitro experiments demonstrated aerolysin's high toxicity to macrophages but not to intestinal epithelial cells. The aerolysin-producing strain was termed Aeromonas sp. macrophage-toxic bacteria (MTB). This finding directly linked a specific bacterial toxin to the loss of a key immune cell population observed in human disease.
Mouse Models Confirm a Causative Role for MTB and Aerolysin. Using mouse models of colonic inflammation, Jiang et al. demonstrated that MTB infection worsened symptoms like weight loss, rectal bleeding, and ulceration. Crucially, genetically engineered MTB lacking the aerolysin-encoding gene lost this pro-inflammatory effect. Furthermore, MTB did not aggravate colitis in mice pre-depleted of gut-resident macrophages. Administering anti-aerolysin antibodies alleviated inflammation in infected mice. These experiments established a causative chain: MTB infection destroys protective macrophages via aerolysin, rendering the colon susceptible to inflammation (see the figure).
Epidemiological Evidence in Human Cohorts. The study extended findings to human populations, analyzing samples from 117 IBD patients and 430 healthy controls across China. Aerolysin-producing MTB was detected in over 70% of UC patients but only ~12% of healthy individuals. Aerolysin was present in the colonic mucosa of all UC patients tested. Notably, MTB was found in only 1 of 38 Crohn's disease patients, indicating a potential specificity for UC. This strong correlative link in humans supports the clinical relevance of the mouse model findings.
Colonization Resistance and the Role of Modern Lifestyle. A key question is why MTB persists in UC patients but not healthy individuals. In mice, MTB could not colonize healthy guts at detectable levels unless colonization resistance was broken by antibiotic pretreatment or existing inflammation was induced. Once established, MTB persisted long-term. This mirrors the hygiene hypothesis and identifies antibiotic use—a hallmark of modern lifestyles—as a major risk factor for IBD. It suggests that dysbiosis or inflammation creates a permissive niche for pathobionts like MTB to thrive and exert their harmful effects.
Revisiting Koch's Postulates for Complex Chronic Diseases. Fulfilling Koch's postulates for multifactorial diseases like IBD is inherently limited. Mice do not spontaneously develop human IBD, preventing the classic test of initiating disease in a healthy host with a pure culture. While Jiang et al. do not fulfill all postulates, they establish a strong causative link in mice and a compelling correlative link in humans. Their work demonstrates how a microbe can be a contributory disease agent by targeting specific host defense mechanisms, a paradigm beyond the original postulates.
Therapeutic Implications and Future Directions. IBD encompasses a complex array of presentations unlikely to stem from a single agent. However, the discovery that microbes can directly target gut-resident immune cells opens new therapeutic avenues. Instead of broadly suppressing immunity with biologics or steroids, targeted strategies could neutralize specific toxins like aerolysin or eradicate pathobionts like MTB. This approach aims to restore gut homeostasis without systemic immunosuppression, representing a promising frontier in personalized IBD treatment.
Conclusion: A Paradigm Shift in Ulcerative Colitis Etiology. The research by Jiang et al. provides a significant advancement in understanding ulcerative colitis pathogenesis. It illustrates how a revised application of microbiological principles can uncover mechanisms where traditional infectious disease models fall short. The role of Aeromonas sp. MTB and its toxin aerolysin highlights the importance of host-microbe interactions and immune cell vulnerability in chronic inflammatory disease, offering new targets for intervention and a refined framework for investigating similar complex ailments.
REFERENCES AND NOTES:
1. S. Falkow, Rev. Infect. Dis. 10, S274 (1988).
2. D. N. Fredericks, D. A. Relman, Clin. Microbiol. Rev. 9, 18 (1996).
3. J. T. Chang, N. Engl. J. Med. 383, 2652 (2020).
4. Z. Jiang et al., Science 390, eadz4712 (2025).
5. T. Kobayashi et al., Nat. Rev. Dis. Primers 6, 74 (2020).
6. A. S. Faye et al., Gut 72, 663 (2023).
7. L. H. Nguyen et al., Lancet Gastroenterol. Hepatol. 5, 986 (2020).
8. J. Sawaed et al., Sci. Adv. 10, eadp4119 (2024).
Date added: 2026-02-14; views: 3;
