Fundamentals of Alloying. Role of Alloying Additions
Unalloyed magnesium, obtained from either the thermal or electrolytic route with different levels of purity, is not used for structural applications. For engineering applications, alloys are created that contain a number of different elements, including Al, Zn, Mn, Si, Zr, Ca, Ag, Li, Cu, alkaline or rare earth elements.
The purpose of alloying additions is to improve strength and other properties of magnesium. Although alloying alters certain properties, the key features of magnesium-based alloys remain similar to those of pure magnesium. The major properties of pure magnesium are listed in Table 1.5.
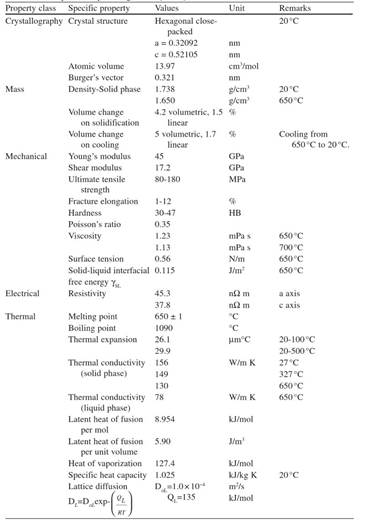
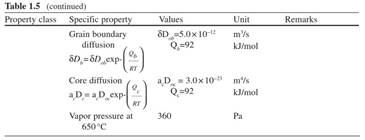
Table 1.5. Properties of pure magnesium
Role of Alloying Additions.Aluminum: Solubility in magnesium reaches 12.7% at 437 °C and is reduced to 3% at 93 °C (Fig. 1.13). In the Mg-rich range, Al forms the Mg17Al12 phase, effective in preserving alloy properties to roughly 120 °C. Additions of Al improve room temperature strength, castability and corrosion resistance. Ductility and fracture toughness are gradually reduced with increasing Al content (Fig. 1.14).
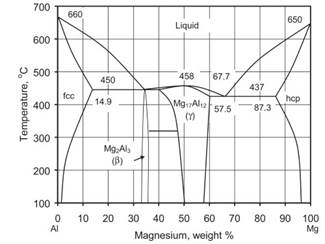
Fig. 1.13. The Mg–Al equilibrium phase diagram
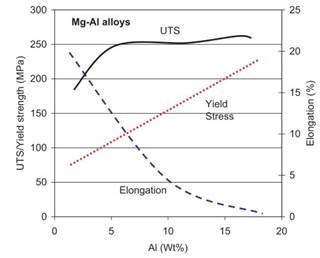
Fig. 1.14. The influence of Al content on mechanical properties of Mg–Al alloys
Zinc: improves room temperature strength through solid solution effect. As shown in Fig. 1.15, the solubility of zinc is 6.2% at 341 °C and 2.8% at 204 °C. In addition zinc also improves fluidity of the melt, but contents above 2% decrease elongation at fracture—especially in the solution-treated condition—and can cause cracking. The ductility reduction is explained through preferential segregation of Zn to the Mg17Al12 phase, thus increasing its volume fraction.
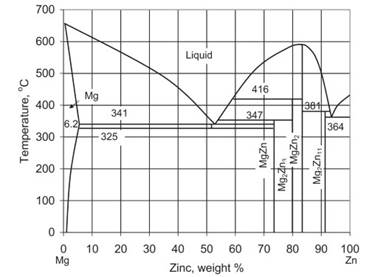
Fig. 1.15. The Mg–Zn equilibrium phase diagram
Manganese: is added to control the iron content. The level of Mn additions depends on the mutual solubility of Fe and Mn in the presence of other alloying elements. Mn increases slightly the yield stress without marked influence on tensile strength.
Solubility of manganese is less than 1% at 482 °C. In alloys containing Al, Mn combines with Al forming MnAl, MnAl4 and MnAl6. All compounds may exist as single particles with the Al/Mn ratio decreasing to the particle center. After heat treatment the MnAl6 phase predominates. The binary diagram Mg-Mn is shown in Fig. 1.16.
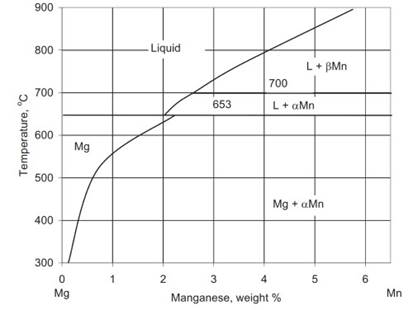
Fig. 1.16. The Mg–Mn equilibrium phase diagram
Silicon: is added to increase the creep resistance by stable silazide precipitates. Addition of Si is limited by the liquid solubility to 1.7% at regular melt temperatures during casting (Fig. 1.17). Additions above 1.16%, which correspond to the Mg-Si eutectic point, cause the formation of coarse, blocky intermetallic particles that do not contribute to improvement in mechanical properties. At the same time Si additions reduce castability.
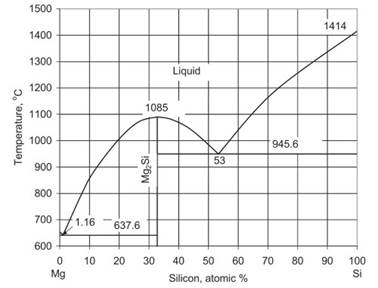
Fig. 1.17. The Mg–Si equilibrium phase diagram
Calcium: is seen as an addition improving the creep resistance and grain size control. Its presence in an alloy reduces general processing behavior, e.g., causing sticking to the mold.
Zirconium: is a strong grain refiner and is added to pure Mg or alloys containing Zn, Ag, Y or Th. It has the same lattice type and nearly identical lattice constant as Mg. It also has a reasonable solubility limit in magnesium. As a result, Zr atoms play a strong role in growth restriction of Mg grains. Zr is capable of diminishing the grain coarsening effect of beryllium. For example, additions of 0.2% Zr to AZ92 contaminated with Be reduces grain size from 510 |jm to 180 |jm [10].
Beryllium: is added routinely in trace amounts in the order of 10-15 ppm to retard oxidation and ignition of molten alloy. It is also effective in removal of iron from magnesium melt: Additions as low as 0.2% allowed reduction of Fe in AZ63 alloy to 2 ppm. Grain coarsening, however, restricts its use for anti-oxidation and anti-ignition purposes. Small Be additions increase the grain size in Mg-Al alloys up to one order of magnitude.
It is suggested that Be might form carbides that are not effective as nucleation substrates for Mg and consume carbon, which could otherwise be used to form particles effective in grain refinement, e.g., Al4C3. According to recent findings, beryllium can poison or considerably degrade any type of heterogeneous nucleus particles existing in melt by coating those particles with surface active surfactant.
Date added: 2023-11-02; views: 527;
