Mass Spectrometry. Amass spectrometer with electrostatic and magnetic sectors
Mass spectrometry is the separation by mass-to- charge ratios (m/z) and the measurement of m/z and abundances of mono- and polyatomic ions in the gas phase. A graphical display of ion abundance as a function of m/z is called a mass spectrum. All mass spectrometers operate under greatly reduced pressure in order to produce and sustain ions in the gas phase.
The British physicist J.J. Thomson observed the first mass spectrum in 1912, which consisted of differences in the trajectories of two isotopic ions of the inert gas neon, subjected to electric and magnetic fields. Near the end of the decade, Thomson’s student at Cambridge University, Francis Aston, developed the first practical mass spectrograph, which was based on the apparatus used by Thomson and employed an ion-sensitive photographic plate as the detector.
During the 1920s and 1930s, other investigators including A. J. Dempster at the University of Chicago and J. Mattauch and R. Z. Herzog in Austria developed mass spectrographs, and Dempster invented the electron ionization technique that is widely used today to produce ions in the gas phase. These early instruments used magnetic and electric fields to separate ions by m/z, and the discoveries made with them had a profound impact on the chemical, physical, and life sciences.
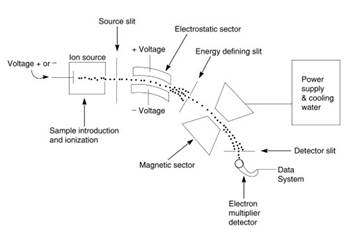
Figure 1. Amass spectrometer with electrostatic and magnetic sectors in the Nier-Johnson double focusing configuration
The Nobel Prize in Chemistry for 1922 was awarded to Francis Aston ‘‘.. .for his discovery, by means of his mass spectrograph, of isotopes, in a large number of nonradioactive elements ...” The atomic weights of all the elements were determined by mass spectrometric measurements of the exact masses and relative abundances of the naturally occurring and man-made isotopes.
Alfred O. C. Nier at The University of Minnesota designed and constructed improved magnetic deflection mass spectrometers during the 1930s and 1940s, and used these instruments to discover new isotopes and to measure the isotopic compositions of many elements including the uranium isotopes. Mass spectrometers were used for analyses of isotopic compositions, measurements of isotopic enrichment, and the production of uranium-235 during the World War II project to develop the atomic bomb.
The Consolidated Engineering Corporation introduced the first commercial mass spectrometer in the U.S. in 1940. This instrument used magnetic deflection to separate ions and was widely employed in the petroleum industry for qualitative and quantitative analyses of gas mixtures during and after World War II.
During the early 1950s Nier and his student E.G. Johnson designed a double-focusing high-resolving power mass spectrometer consisting of tandem electrostatic and magnetic sectors and the electrical detection of ions rather than photographic plates. The Nier-Johnson design was used in many commercial spectrometers, and became a standard instrument for exact measurements of the m/z of elemental and polyatomic inorganic and organic ions.
During the late 1940s and 1950s, mass spectrometer designs began to appear that were not dependent on heavy, power-consuming electromagnets. In 1955 W.C. Wiley and I.H. McLaren of the Bendix Aviation Corporation described a time-of-flight (TOF) mass spectrometer, and the Bendix Corporation marketed instruments based on their design throughout the 1960s. In a TOF mass spectrometer, ions are accelerated in batches into an evacuated flight tube by a rapid series of high potential pulses at the acceleration electrode.
The time of flight of ions from acceleration to detection is a function of m/z. However, the resolving power of vintage 1960s instruments was still too low for most applications. During the 1980s several design innovations, including the electrostatic ion mirror developed by B. A. Mamyrin and D. V. Shmikk in Russia, and very fast semiconductor electronics gave significantly improved TOF mass spectrometer performance. The TOF mass spectrometer is widely used, especially for the characterization of large molecules of biological origin and in experiments where very high-speed data acquisition is required.
Another innovation was the linear quadrupole mass spectrometer, or mass filter, described by W. Paul and H. Steinwedel of The University of Bonn in 1953. Radio frequency and direct current potentials are applied to four parallel electrodes positioned at the apices of a square and, depending on the potentials, ions of specific m/z pass through the center of the quadrupolar field to the detector while all other ions are deflected and discharged on the rods.
The linear quadrupole is the most widely used type of mass spectrometer, especially when mass spectrometry is combined with gas and liquid chromatography (GC and LC) sample introduction. It is compact in size, low in weight, relatively low in cost, and has a tolerance to higher pressures—about 10-5 Torr—than most other types of mass spectrometers. However, it does not have high resolving power or exact m/z measurement capability.
The ion trap mass spectrometer is a three-dimensional quadrupole in which ions are stored for milliseconds by a radio frequency potential applied to a ring electrode. Ions are ejected from the trap according to their m/z by increasing the amplitude of the radio frequency potential. Wolfgang Paul was awarded the Nobel Prize in Physics in 1989 for the inventions of the linear quadrupole and the quad- rupole ion trap. The ion trap mass spectrometer is widely used in GC/MS and LC/MS applications.
The Fourier transform mass spectrometer (FTMS) was derived from the ion cyclotron resonance spectrometer that was introduced commercially by Varian Associates in 1966. In 1974 M. B. Comisarow and A. G. Marshall of the University of British Columbia developed the FTMS which uses a strong magnetic field, typically from a superconducting magnet, to trap ions and cause them to spiral towards the detector at circular frequencies that are related to their m/z.
Unlike other mass spectrometers, ions are not physically separated, but are detected by measuring an image current induced in the walls of the trap. The m/z of the ions are determined by Fourier transforms of the image current data. The FTMS requires a very low operating pressure; that is, 10-8 Torr or lower, but is capable of very high resolving power and exact mass measurements.
Samples for mass spectrometry may be gases, liquids, or solids but their components must be converted into gas-phase positive or negative ions before mass analysis. Many types of sample introduction systems are used, and sample introduction and ionization may be separate or combined in a single process. Ions are injected from the sample introduction/ionization source into the spectrometer for very rapid analysis that is typically on a microsecond or shorter time scale.
Ions may be formed and injected continuously, pulsed into the spectrometer in batches, or introduced and stored in batches. Two mass spectrometers are often arranged in tandem configurations to facilitate special experiments, for example, the separation of an ion of specific m/z in the first spectrometer, collision-induced dissociation of the ion, and analysis of the decomposition products in the second mass spectrometer.
All mass spectrometers are very sensitive and produce measurable signals from quantities of sample in the range of micrograms (10-6) to femtograms (10-15) or less. Nearly all commercial mass spectrometers produced during the last third of the century incorporate dedicated digital computers that control the operations of the mass spectrometer, acquire and store mass spectro- metric data in digital form, and process or display the m/z and abundance data. Operations of most types of mass spectrometers, especially the TOF, quadrupole ion trap, and FTMS, would be impossible without very fast digital computer technology.
The major application of mass spectrometry is the determination of chemical composition and structure in many fields of investigation including agriculture, beverages and foods, biological systems and processes, the environment, geology, industrial materials, petroleum exploration and processing, pharmaceutical discovery, natural products, and space exploration.
Date added: 2023-11-02; views: 510;
