Microscopy, Optical. Basic Compound Microscope
A microscope is an optical instrument consisting of a sequence of lenses, the purpose of which is to overcome the limit imposed by the least distance of distinct vision of the human eye, typically 25 centimeters. At this distance, the eye cannot see objects smaller than about 0.1 millimeters; 0.2 millimeters can be seen without effort. By placing a single lens, a magnifying glass, in front of the eye, the object being examined can be brought much closer and the eye then sees a distant virtual image, which is an enlarged representation of the object.
The magnification M is given approximately by 25/f, where f is the focal length of the lens (in centimeters). It was with such a primitive microscope that Anton van Leeuwenhoek made his celebrated observations of organisms in water and spermatozoa in the seventeenth century. When higher magnifications are required, a second lens is added (Figure 7); this two-lens design, with an objective lens close to the specimen and an eyepiece, is the basic form of the compound microscope, invented around 1610 by Galileo. The magnification is now given by -(25f) (v/u), where the distances v and u are defined in the figure (the minus sign indicates that the image is inverted).
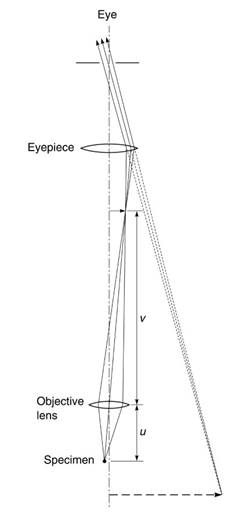
Figure 7. Basic compound microscope
The design of the objective lens requires considerable care and skill since a simple glass lens suffers from defects known as aberrations, associated with the steep inclination of some of the rays (spherical aberration, giving spatial distortion) and the spread of colors in white light (chromatic aberration). These defects can be eliminated or kept acceptably small by a suitable choice of the shapes of the lens surfaces and of the type of glass employed.
The specimen is placed on a glass slide and may be protected by a glass cover slip. The specimen stage can usually be rotated and translated. For opaque specimens, the microscope may be equipped with an epiilluminator, a source that shines light from above the specimen; light reflected from the surface then generates the image.
It might seem from the above geometrical arguments that there is no limit to the magnification of a microscope. Although it is true that extra lenses could be added to attain very high values of the magnification, it was shown by Ernst Abbe of Zeiss in the nineteenth century that there is a limit beyond which no further fine detail can be discerned in the image. The microscope has a limit of resolution, a consequence of the wave nature of light.
This limit is given by k λ NA), in which k is a constant (k ≈ 0.6), λ denotes the wavelength of light (about 0.6 micrometers in the middle of the visible spectrum), and NA is the ‘‘numerical aperture,’’ a quantity that measures the light-gathering ability of the objective lens and is determined by the largest angle ‘‘seen’’ by the objective (the so-called angle of acceptance) and the refractive index of the medium between the specimen and the lens surface; in order to increase this refractive index, oil may introduced between the lens surface and the specimen.
The numerical aperture is at best about 1.45. In practice, the resolution can reach about 0.2 micrometers with an oil-immersion objective and about 0.3 micrometers without oil. The useful magnification is hence about 1200 and 800 times, respectively; increasing the magnification will reveal no further detail.
The contrast seen in the image usually arises from absorption of the light incident on the specimen by opaque features of the latter. The amplitude of reflected light is thus reduced by varying amounts, depending on the opacity, and these variations in amplitude are perceived by the eye as the image of the specimen. However, some biological specimens are largely transparent and generate no amplitude contrast. They may nevertheless be of interest because of thickness variations or changes in the refractive index of the material of which the object is composed.
Such specimens are said to alter the phase of the illuminating beam but not its amplitude and special arrangements have been devised to create ‘‘phase contrast’’ from such objects. In order to explain how this is achieved, we must first introduce the notion of coherence. Light sources are typically large and produce white light, which contains a broad range of visible wavelengths. Such sources are said to be incoherent because the light emitted form any point on the source is unrelated to that from any other point.
At the other extreme are point sources emitting a very narrow range of wavelengths; these are both spatially (small emitting area) and temporally (narrow wavelength spread) coherent and it is such sources that are required for phase-contrast microscopy, discovered by Frits Zernike in the 1930s. Here, part of the light from the specimen passes through a phase plate, a thin layer of glass, the thickness of which is chosen in such a way that the phase of the light passing through it is altered by a suitable amount. This phase-shifted beam is recombined with the other part of the light beam and the result is that the phase differences, invisible to the unaided eye, are converted into amplitude (or brightness) variations. For this invention, Zernike was awarded the Nobel Prize in 1953.
The basic form of the microscope may be modified and extended in many ways. Frequently, a binocular eyepiece is added, and the microscope tube may be duplicated to give stereoscopic effects. From the 1960s it became common for lenses to be coated, for example, to reduce glare. It is usual today to add a recording medium, film or a numerical device, on which individual images or sequences can be captured.
In the 1970s it became possible to capture microscopical images with a television camera, and then to digitize them. Images recorded in numerical form are often transferred to a computer for subsequent analysis or processing. For the study of crystals, particularly minerals in thinly cut rock sections, the incident illumination may be polarized by means of a Nicol prism, the polarizer (a natural rhomb of calcite, cut in two parts, which are then cemented together with Canada balsam). The light emerging from the specimen is analyzed by means of a second Nicol prism (the analyzer).
Polarized light microscopy (often called petrographic microscopy) gives valuable information about the boundaries between mineral grains and can be used for identification because the refractive index of many crystals is not the same in all directions (anisotropy). Polarized light selects a particular direction and, on rotating the specimen, different amounts of light will be transmitted, depending on the relative orientations of the crystal and the direction of polarization.
In a confocal microscope the optics are designed in such a way that information is gathered only from a very thin slice of the object, rejecting out of focus light from other points of the specimen. A screen with a pinhole at the other side of the lens removes all rays of light not initially aimed at the focal point. The specimen is illuminated by this spot of light and the specimen scanned point by point. There is never a complete image of the sample—at any given instant, only one point of the sample is observed and the detector builds up the image one pixel at a time.
Scanning optical microscopes (SOM) had been proposed in 1928 by Edward H. Synge, and first built in 1951 by John Z. Young and F. Roberts. The performance of a SOM was improved when in 1955 Marvin Minsky invented the confocal scanning microscope. The resolution of Minsky’s confocal microscope was up to twice that of a simple SOM.
The phenomenon of fluorescence has given rise to the family of confocal fluorescence microscopes. A few specimens emit light (or fluoresce) naturally but the fluorescence microscope exploits the fact that other specimens can be ‘‘stained’’ with fluorescent dyes, the colors of which correspond to different features of the object. Fluorescence microscopes in use today follow the basic design of Johan S. Ploem from the 1970s. Light from a laser (i.e., very high intensity) is directed onto the specimen by means of a dichroic mirror, a plate that reflects light of all wavelengths below a threshold value and transmits all wavelengths above this threshold.
This incident light causes the dye in the specimen to fluoresce at a color with a longer wavelength; a small pinhole in the image plane selects the fluorescence signal, which is then recorded. Such an optical arrangement is very sensitive to the exact level in the specimen from which the fluorescence emanates. In order to form a full image of the specimen, scanning mirrors are added (Figure 8), with which the beam scans the specimen in a raster pattern (like the spot on a television screen); these mirrors also cancel the scanning effect on the return beam.
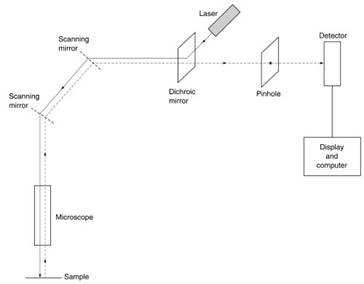
Figure 8. Confocal microscope
The latter is intercepted by the pinhole and the signal that passes through the opening falls on the detector, which records an image of a particular layer of the specimen. By focusing on successive layers, a full three-dimensional image can be built up.
Another new member of the microscope family is the scanning near-field optical microscope (SNOM), a close relative of the atomic-force microscope, which provides information far beyond the traditional resolution limit defined earlier. The SNOM was independently developed by Dieter W. Pohl and others at IBM in Zurich and Aron Lewis at Cornell University. Here, a sharp conical optical fiber tip is placed very close to the surface of the specimen.
The diameter of the tip and the distance between tip and surface are much smaller than the wavelength of light, typically 50 nanometers or less. The surface is then displaced systematically and the tip is raised or lowered to ensure that the tip-surface distance remains constant. Two signals are recorded: the intensity of the light reflected from the surface (or fluorescence excited in the sample) and the vertical movement of the tip. The first is used to form the SNOM image while the second provides topographic information about the surface. Very many variants on this basic design have been developed, notably a transmission version for transparent specimens.
Date added: 2023-11-02; views: 565;
