Modes of HIV Transmission: Rates and Risk Factors
Understanding the different modes of transmission and appreciating associated risk factors play an essential role in grasping the epidemiology of the HIV/AIDS epidemic. HIV may be transmitted via sexual intercourse (either male to female, female to male, or male to male), via IDU, vertically from mother to child, through transfusions of blood or other blood products, or via occupational exposure to HIV-infected bodily fluids.
Sexual Transmission. Sexual transmission accounts for the 75-85% of HIV infections worldwide and heterosexual intercourse accounts for the vast majority of these transmission events (Royce et al., 1997). The likely explanation for this is that heterosexual transmission predominates in those regions of sub-Saharan Africa with the highest HIV prevalence, rather than that heterosexual intercourse is a more efficient means of transmission.
The average rate of infection for vaginal intercourse is estimated to be 0.0011 per contact and several studies suggest vaginal transmission from an infected male to an uninfected female is more efficient than when the female partner is HIV-infected (Figure 5) (Gray et al., 2001). This is in comparison to significantly higher rates for unprotected anal sex, which is the most efficient mode of sexual transmission and has an average rate of infection of 0.0082 per contact (Vittinghoff et al., 1999).
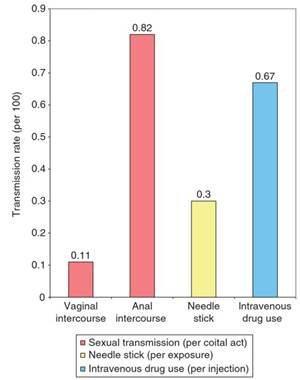
Figure 5. Rates for different modes of transmission
One of the most significant and well-established risk factors in sexual HIV-1 transmission is the quantity of district of Uganda, one study demonstrated a significant dose-response relationship between increased HIV-1 RNA viral load in plasma and increased risk of transmission. For every log10 increment in the viral load, there was a 2.5-fold increase in HIV-1 transmission and no transmission occurred in this cohort when the infected partner had a plasma HIV-1 RNA level less than 1500 copies per milliliter (Quinn et al, 2000).
The highest HIV-1 viral load occurs in the setting of end-stage AIDS and during primary HIV infection, making these periods of high transmission risk. The latter is a particular concern from a public health standpoint because when a person is first infected with HIV, he or she may not know his or her HIV status, and may unknowingly spread HIV at high rates.
A study in Uganda demonstrated that the highest HIV transmission rates were within 2.5 months after seroconversion of the infected partner, which represents primary infection (0.0082 per coital act), and during the period 6-26 months before the death of the infected partner, which represents advanced AIDS (0.0028 per coital act). The average rates of HIV transmission were 0.0007 per coital act among chronically infected partners (Wawer et al., 2005).
Genital shedding of HIV-1 may also increase HIV transmission risk and some have suggested that it may be a better predictor of HIV transmission than systemic HIV-1 viral load (Baeten and Overbaugh, 2003). A number of studies have reported strong correlation between plasma and genital tract HIV-1 levels; however, one published study has found a significant association between genital HIV shedding and increased transmission (Pedraza et al., 1999).
Another factor that appears to increase risk of transmitting HIV, as well as risk of acquiring HIV in the exposed, uninfected partner, is the presence of sexually transmitted infections (STIs). STIs may facilitate HIV shedding in the genital tract, cause local recruitment of susceptible inflammatory cells, or disrupt genital mucosal surfaces. Increased risk of both HIV transmission and acquisition has been shown for both ulcerative and nonulcerative STIs. Early studies identified syphilis as increasing risk of acquisition 3.1- to 12.8-fold (Otten et al., 1994).
A separate study found a fivefold increase in acquisition of HIV in the presence of genital ulcers, where 89% of the ulcers were chancroid (Cameron et al., 1989). The ulcerative STI that has received the most attention recently has been herpes simplex virus type 2 (HSV-2). Two meta-analyses have demonstrated that having HSV-2 increases the risk of transmission approximately threefold (Freeman et al., 2006) and increases risk of HSV-2 acquisition twofold (Wald, 2004).
The association between HSV-2 infection and HIV systemically and in genital secretions has been firmly established in a recent trial in Burkina Faso which showed that HSV suppression therapy with valacyclovir significantly reduced genital and plasma HIV-1 RNA level in women who are coinfected with HSV-2 and HIV-1 (Nagot etal, 2007). Randomized clinical trials (RCTs) testing the hypotheses that HSV-2 suppression will reduce risk of HIV transmission and acquisition are underway.
In terms of nonulcerative STIs, several studies have shown significantly increased risk in the acquisition of HIV in the presence of gonorrhea, chlamydia, trichomoniasis, and bacterial vaginosis (Plummer et al., 1991; Laga et al, 1993; Craib et al., 1995; Sewankambo et al, 1997; Taha et al., 1998). These data and others led to four community-level RCTs conducted in Mwanza, Tanzania; Rakai, Uganda; Masaka, Uganda; and Manicaland, Zimbabwe to see the effect of STI treatment on HIV transmission.
The study in Mwanza showed a 38% reduction in HIV incidence among those receiving the intervention to prevent STIs when compared with the nonintervention group (Grosskurth et al., 1995); however, none of the other three studies was able to demonstrate a positive impact of STI treatment on HIV transmission (Wawer et al., 1999; Kamali et al., 2003).
The main explanation for these discordant findings is that Mwanza represented a community that was in an early phase of the HIV epidemic, while the other three communities were in a more mature phase of the epidemic (Korenromp et al., 2005). Therefore, new HIV epidemics characterized by high-risk behavior may benefit significantly more from interventions than HIV epidemics in a more mature phase.
Vaginal microbicides targeting STIs and HIV have also been evaluated for a protective effect against HIV transmission. Nonoxynol-9 has been studied in several RCTs, and to date, none has shown any benefit against HIV transmission (Kreiss et al., 1992; Wilkinson et al., 2002). In fact, some of these studies have demonstrated increased risk of HIV transmission among those women using the microbicide, probably secondary to increased genital inflammation and ulceration. Such risks are being closely monitored in ongoing RCTs examining other microbicides to determine their impact on HIV transmission.
Hormonal contraception is another potential risk factor for HIV acquisition that has been extensively studied. Hormonal contraception could increase a woman’s susceptibility via hormonally induced changes in the vaginal mucosa or through associated cervical ectopy (Chao et al., 1994; Daly et al., 1994; Sinei et al., 1996). However, recent studies, including a trial following 6109 women in Uganda, Zimbabwe, and Thailand, have not found an association between hormonal contraceptive use and HIV acquisition (Mati et al., 1995; Morrison et al., 2007; Myer et al, 2007).
Increased transmission rates have also been associated with specific sexual practices and several partnership factors. The different modes of sexual intercourse differ in their efficiency of HIV transmission, with anal intercourse having the highest rates of transmission, followed by vaginal, and then oral sex (Royce et al., 1997).
In addition, traumatic sex (fisting, rape), sex while under the influence of alcohol or cocaine, sex during menses, sex during pregnancy, the use of vaginal desiccants, and the use of vaginal tightening agents have been associated with increased HIV transmission risk in several studies (Lazzarin etal, 1991; Henin etal., 1993; Seidlin etal, 1993). Partnership factors that increase HIV transmission risk include high number of partners, high frequency of sexual contact, and concurrency. Concurrency is a partnership timing factor defined as being engaged in more than one sexual relationship at a single time point. In contrast, serial monogamy occurs when each partnership ends before the start of the next sexual relationship (Morris and Kretzschmar, 1997).
One important intervention to prevent HIV transmission is condom use. In several longitudinal studies, consistent condom use was 80-87% effective in preventing HIV transmission (Davis and Weller, 1999; Weller and Davis, 2002). Condom use is also associated with decreased transmission of gonorrhea, chlamydia, and HSV-2, all important HIV risk factors (Shafii et al., 2004). Despite these compelling data, increasing condom use within at-risk populations has been a challenge. Several behavioral studies designed to reduce risky behavior and increase condom use have had no impact on HIV incidence (Hearst and Chen, 2004).
Strong data also support circumcision of adult men as a means to protect against HIV transmission. A number of observational studies have demonstrated a significant association between male circumcision and HIV transmission (Quinn etal., 2000). These were recently validated in three large clinical trials, which randomized men to circumcision or no circumcision. One biological explanation is that keratinization of the glans when circumcised prevents tears and abrasions and decreases the presence of Langerhans cells and other HIV target cells (Quinn, 2006).
The first RCT was completed in South Africa and showed 60% protection against HIV infection in the intention to treat analysis and 75% protection in the per protocol analysis (Auvert et al., 2005). Two other concurrent RCTs in Kenya and Uganda were recently published and confirmed that male circumcision reduced HIV incidence in men with similar risk reduction as the RCT in South Africa (Bailey etal, 2007; Gray et al, 2007).
Date added: 2024-02-18; views: 876;
