Plants as Poikilothermic Organisms. Temperature Dependence of Metabolism
Only very few examples of plant organs that can produce heat by alternative respiration are known. One of these is the spadix of Aracean flowers. Plants are poikilothermic organisms, which cannot maintain their temperature at a level different from that of their immediate environment. However, as the exterior temperatures can vary substantially for different organs such as roots (soil temperature) or leaves (air temperature, local heating by sunrays), temperatures within one individual plant can be very different at any given time. Furthermore, environmental temperatures can change rapidly. Thus, plant cells, tissues and organs require the ability to autonomously respond to temperature fluctuations.
Temperature Dependence of Metabolism. In contrast to homeothermic organisms, which maintain a stable inner temperature, plants need to balance the biochemical reactions of their metabolism. This is not a trivial task, as the rate even of an enzyme-catalysed reaction depends on the temperature, just like any non-catalysed reaction (with the only difference being that the enzyme lowers the activation energy). The simple measure for the temperature dependency of a reaction is the so-called Q10 (the quotient of the rates (V) of a reaction at two temperatures (T) differing by 10 K):

An increase in the temperature speeds up the reaction; a decrease slows it down. The Q10 is a direct indicator of the temperature dependence of a reaction. The Q10 for enzymatic reactions lies between 1.4 and 2.5, while for biophysical processes it is between 1.03 and 1.3. Both the range of Q10 values for enzymatic reactions and the difference between biochemical and biophysical reactions pose fundamental problems for plants.
When the rate constants, kT1 and kT2, are inserted instead of the rate (V) of the reaction, the activation energy (Ea) can be calculated:
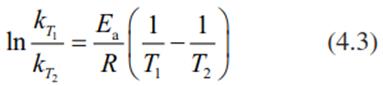
where R is the universal gas constant.
Because of variations in the efficiency of catalysis, not all biochemical reactions that take place at the same time in a plant cell require the same activation energy. Therefore, a change in the temperature could easily disturb the metabolic balance in reaction chains—in particular, those involving steps with a high Q10. Plants must be able to compensate for potential temperature-caused metabolic imbalances—that is, changes of pool sizes of metabolites.
The challenges arising from the much lower temperature dependence of biophysical reactions apply to photosynthesis in particular. Absorption of light energy is barely affected by temperature, while the CO2 fixation in the Calvin cycle is substantially slower at cooler temperatures. This leads to an excess of energy, which has to be dissipated in order to prevent injury (Fig. 4.2).
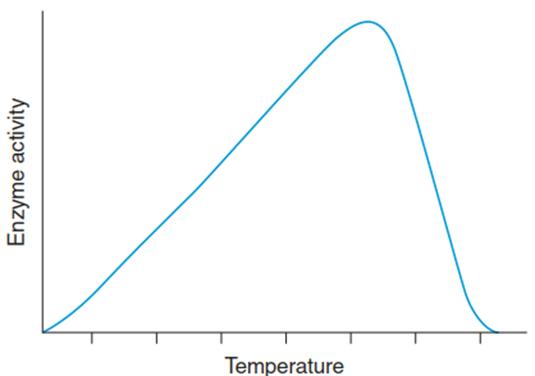
Fig. 4.2. Temperature dependence of an enzyme-catalysed reaction. While at temperatures below the optimum the Q10 rule is applicable, temperatures higher than the optimum progressively inactivate the enzyme by affecting its molecular structure (denaturation). Therefore, the temperature response curve of a biochemical reaction is asymmetrical with a strong decline towards higher temperatures
Since the cellular metabolism is composed of a large number of individual reactions whose reaction rates are dependent not only on the temperature but also on the equilibrium constants and sizes of the substrate pools, heat inactivation of the cellular metabolism is not as sudden as that of individual enzymes. Comparably strong inactivation does not take place at temperatures below the optimum.
Date added: 2025-01-17; views: 321;
