Membrane Fluidity. Freezing
In addition to its influence on the rates of metabolic processes, temperature has a strong effect on cellular membranes. Biomembranes consist of a bilayer of bipolar lipids with embedded proteins such as transporters, ion channels or receptors, as well as photosynthetic and respiratory multi-protein complexes. Their structure is described in the fluid mosaic model (see biochemistry textbooks such as Buchanan et al. 2015; Heldt and Piechulla 2010).
This lipid bilayer has to maintain a fluid state for functionality. Also, signal transduction events and localised responses of cells require horizontal mobility of proteins in a membrane or the production of signal compounds from membrane lipids. The fluidity of these lipids depends, in the first approximation, on the solid (crystallised) or liquid state of the fatty acids which, at a given temperature, depends on the chain length and the degree of unsaturation. The longer the chain length and the smaller the number of double bonds, the higher the melting point (Table 4.1).
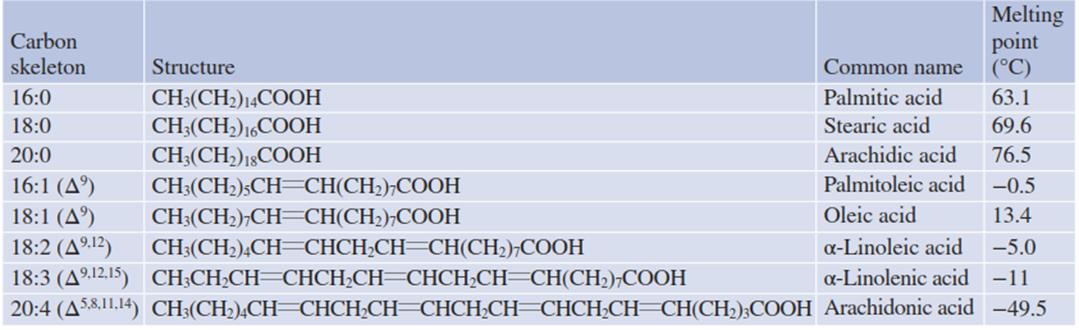
Table 4.1. Chemical structures and melting points of the most common fatty acids in biomembranes
Polyunsaturated fatty acids need more space than saturated fatty acids because of the rotational movement of the carbon chain at the double bonds. Therefore, membranes with a high proportion of unsaturated fatty acids are less tightly packed and hence more fluid than those where saturated fatty acids dominate. On the other hand, the thickness of the lipid bilayer decreases with an increasing proportion of unsaturated fatty acids.
Freezing. A fourth temperature-related stress phenomenon—besides metabolic imbalances, changes in membrane fluidity and heat denaturation of proteins—is the freezing of water (i.e. the formation of ice crystals) within an organism (Boxes 4.1 and 4.2). This represents a severe additional threat. Thus, chilling stress (exposure to low positive temperatures) is distinguished from freezing stress (exposure to sub-zero temperatures) (Fig. 4.4).
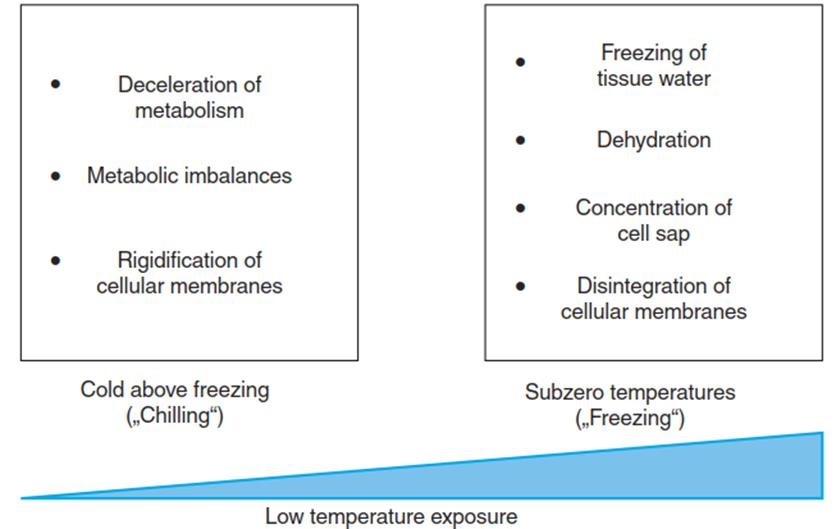
Fig. 4.4. Stress from cold and frost at the cellular level. Low temperatures below the optimal range but above the melting point of water (= chilling) slow down enzymatic reactions. Because these are not equally affected by temperature changes (i.e. they have different Q10 values), metabolic imbalances (Jones et al. 1998; Janska et al. 2010) occur. Cellular membranes rigidify as a result of reduced movement of lipids. A further decrease in temperature below the melting point (= freezing) can lead to the freezing of extracellular water. This causes dehydration because of a massive reduction in water availability owing to crystallisation. Cells lose water to the exterior and cell sap becomes more concentrated. Intracellular formation of ice crystals causes disintegration of cellular membranes and thereby ion leakage
Massive injury can occur because of severe dehydration upon formation of ice crystals (Box 4.2). Ice crystals predominantly build up in the apoplast because of the lower concentration of solutes relative to the cytosol. Ice formation in the apoplast leads to a drastic drop in water availability. The concomitant decrease in the water potential to extremely negative values causes damage to membranes and in turn the cellular compartmentation. Ice, in contrast to liquid water, is hydrophobic, and ice crystals at the surface of a biomembrane cannot exert the membrane-forming forces required for the formation of a lipid bilayer.
Amphiphilic lipids unite with micelles or membranes only when they are in a sufficiently hydrophilic medium—that is, stabilised by an ordered water film. If this film disappears, the stabilising effect of the hydrophobic interaction vanishes and the lipids aggregate into droplets. This is then called the “lipid hexagonal II phase” (Fig. 4.5).
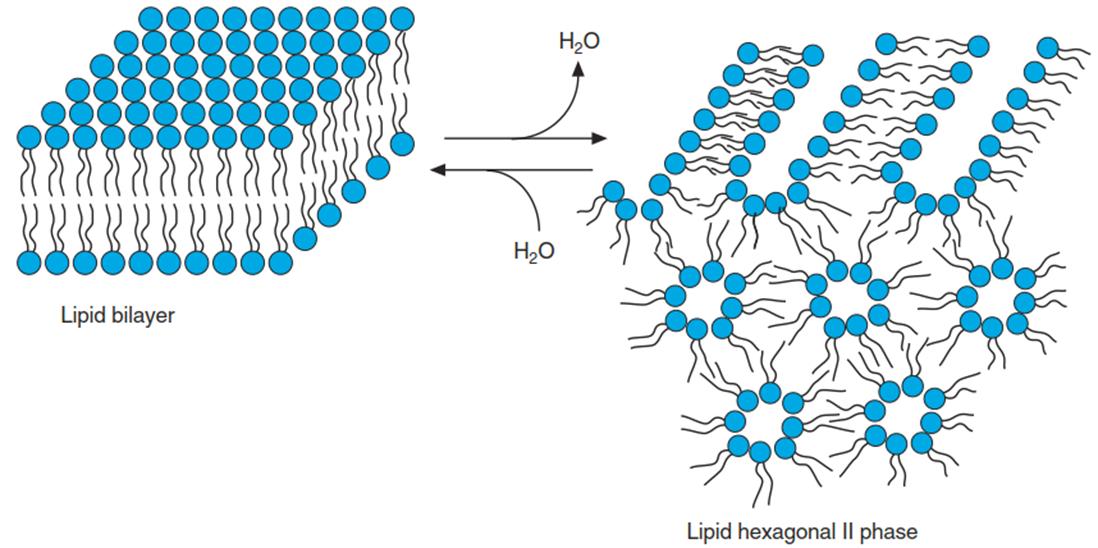
Fig. 4.5. Disintegration and reconstruction of a phospholipid bilayer by water removal (e.g. freeze dehydration) and rehydration. Phospholipids form lipid droplets or threads in the hexagonal II phase. (Modified from Crowe et al. (1983))
Thus, intracellular crystallisation of water, which inevitably brings ice into contact with biomembranes, results in membrane disintegration and collapse of the cellular compartmentation. Loss of membrane integrity is indicated by electrolyte leakage, a parameter commonly used to quantify freezing tolerance (Box 4.3). Furthermore, freezing of water in xylem vessels and subsequent thawing causes air breakage of the water column, called an embolism.
Date added: 2025-01-17; views: 388;
