How Much of the Tissue Water Can Freeze?
Liquid water can be distinguished from ice by nuclear magnetic resonance (NMR) spectroscopy. The frozen and the still liquid portions of the water content of a leaf, for example, can thus be determined by running the NMR scans at a series of sub-freezing temperatures. Plotting the portion of liquid water versus the temperature produces so-called freezing curves (Fig. 4.7).
The lower the temperature, the more cellular water is deposited as ice in the intercellular cavities. The nearly hyperbolic freezing curve of ivy (Hedera helix) can be transformed into a straight line by plotting the residual liquid water against the reciprocal of the temperature. The line cuts the y axis at a value, K, which is the amount of water that, because of its binding to macromolecules, cannot freeze. In the example shown in Fig. 4.7, K ≈ 5%.
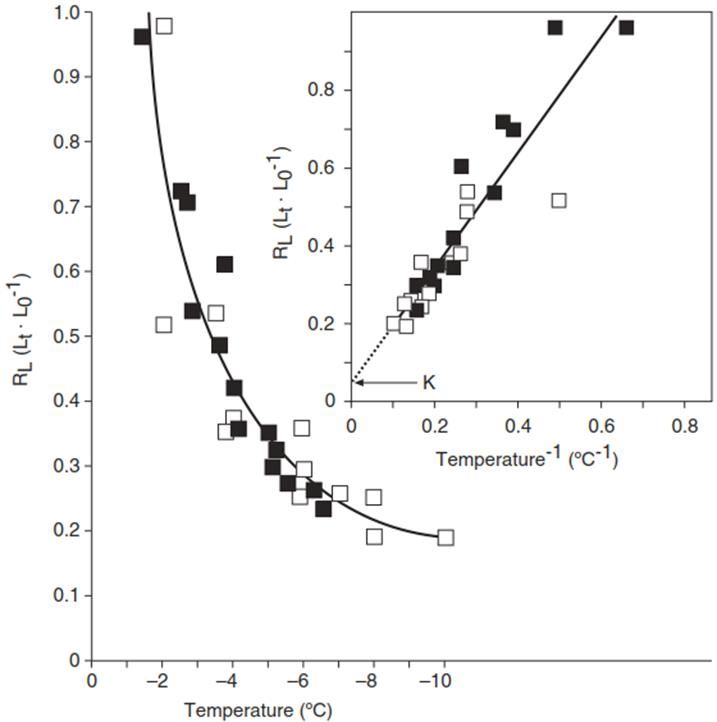
Fig. 4.7. Freezing curve of frost-hardened ivy (Hedera helix) leaves. The curve shows the liquid water proportion (RL) of the total water content (L0) as dependent on the frost temperature (t)

Upon ideal equilibrium freezing, a rectangular hyperbola is obtained, which can be transformed into a simple Linear relationship (insert). The point at which this line cuts the y axis is the K point, indicating the non-freezable portion of tissue water (Hansen and Beck 1988)
Interestingly, at a normal winter temperature of -10 °C, about 80% of the water in an ivy leaf is already frozen outside the cells and accordingly shrinkage of the cell volumes is dramatic and can result in considerable negative turgor (Zhu and Beck 1991). The concomitant increase in the concentration of the intracellular solutes is likewise dramatic (e.g. fivefold in the presented example). A highly concentrated solution has a substantially lower freezing point than the original cellular solutions, thus counteracting intracellular ice formation.
Date added: 2025-01-17; views: 334;
