Measurement of Cold Hardiness and Damage by Cold and Freezing of Plant Tissue
The degree of cold/frost hardiness of a plant or plant tissue can be experimentally examined by subjecting the sample to a so-called freeze-thaw cycle with subsequent quantification of the induced damage. To warrant full equilibration of the sample with the applied temperature, low rates of cooling and rewarming must be applied (Wisniewski et al. 2014). A frequently used rate is 2 K/h. The protocol for a freeze-thaw cycle is shown in Fig. 4.11.
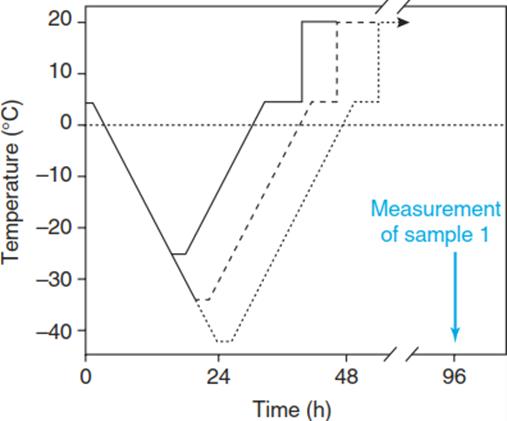
Fig. 4.11. Temperature course of a freeze–thaw cycle experiment. In order to determine the degree of cold hardiness or damage, the samples must be cooled to several defined final temperatures, where they remain for 2 h. Rewarming then occurs at the same rate as cooling. After rewarming, the sample is kept for 5 h at +5 °C and subsequently for 24 h or even longer at ambient temperature
For quantification of the damage, which can result from cooling as well as from rewarming, the sample is kept after rewarming at room temperature for at least 24 h to allow development of potential damage. Commonly used assays for the degree of damage and cold resistance, respectively, are biochemical activity tests such as the triphenyl-tetrazolium test for dehydrogenase activity or the electrical conductivity test for membrane leakage (Fig. 4.12).
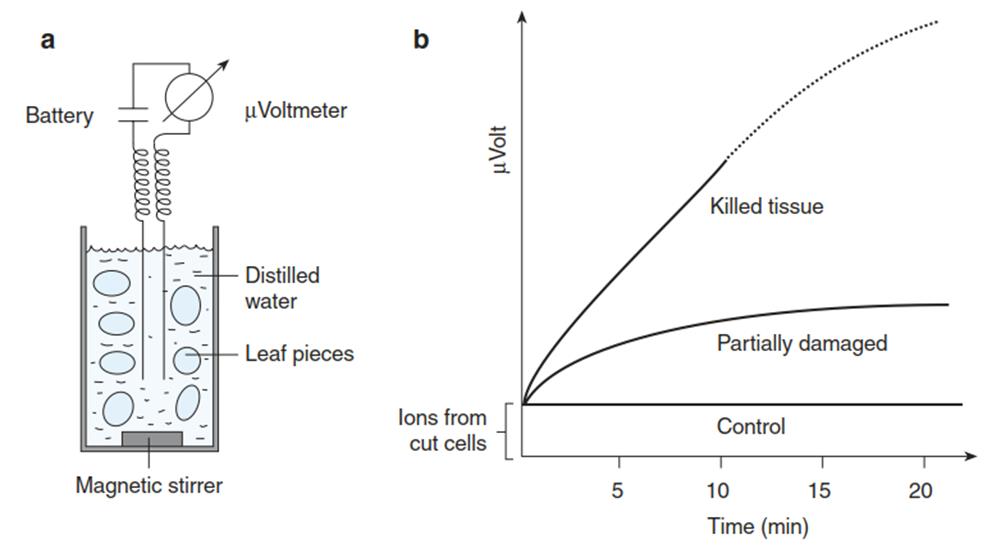
Fig. 4.12. Electrical conductivity assay. Damage to plant tissues leads to loss of selective membrane permeability - that is, inactivation of ion channels and transporters in plant membranes. Consequently, solutes leak out of the tissue. When, for example, excised leaf discs are floated in distilled water a, low molecular weight compounds diffuse into the medium, among them several ions. The increase in the conductivity of the water can be measured conductometrically. The more cells have been damaged, the higher the resulting conductivity is b
Controls are undamaged as well as completely killed (by dipping in liquid nitrogen) samples. Cold hardiness is commonly expressed as the LT50 - the temperature at which 50% damage (e.g. ion leakage of 50%) becomes apparent.
Cold Acclimation and Freezing Tolerance. A complex array of cellular mechanisms (a cold tolerance syndrome) counteracts the negative consequences of exposure to low non-freezing temperatures and to temperatures below 0 °C. The efficiencies and relative contributions of different mechanisms vary between plant species, as well as depending on the mode of stress (e.g. acute versus chronic freezing stress).
Also, the amount of direct evidence supporting the function of a particular mechanism for survival at low temperatures varies. Clearly of central importance is the adjustment of membrane fluidity through the modulation of lipid compositions. Furthermore, metabolic imbalances causing, for instance, the risk of photoinhibition need to be buffered. Various proteins and metabolites that protect cellular structures are synthesised during cold acclimation.
With respect to stress caused by sub-zero temperatures, mechanisms can be divided into tolerance and avoidance strategies—that is, those enabling plants to endure the formation of ice crystals versus those suppressing or even promoting the formation of ice crystals. Several reactions upon freeze dehydration resemble those observable during drought or salinity stress.
At the molecular level, signal transduction networks activated by the three different types of stress target partly the same genes because some protective mechanisms are the same (Yamaguchi- Shinozaki and Shinozaki 2006; Huang et al. 2012) (Chap. 2). This explains the so-called cross-protection: exposure to drought improves cold tolerance, and vice versa. The same applies to osmotic strain caused by salt.
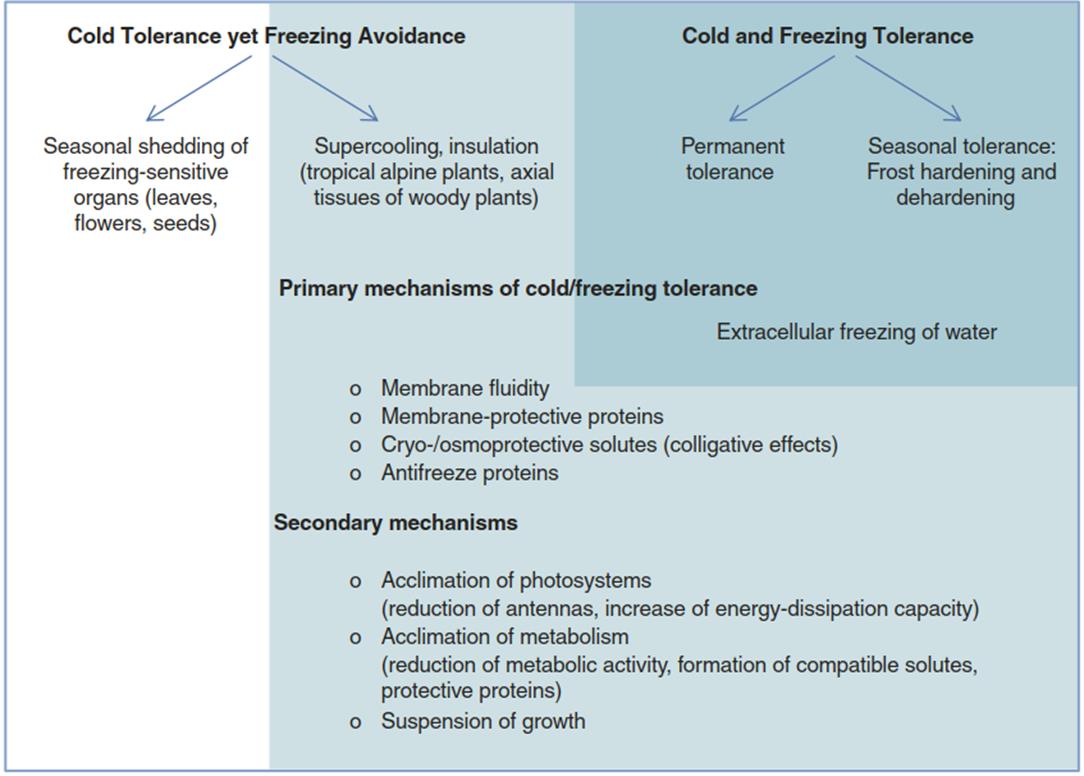
Fig. 4.13. Mechanisms that allow plants to survive in habitats characterised by permanently, transitorily or seasonally occurring cold and frost
Avoidance mechanisms involve synthesis of so-called antifreeze proteins, which inhibit the growth of ice crystals, or other measures to at least partially suppress the formation of ice crystals (Moffatt et al. 2006) (Fig. 4.13).
Date added: 2025-01-17; views: 352;
