Herbivory. Constitutive Defences
Insects represent the most species-rich group of organisms, and a large number of them (an estimated one million species or more) are herbivorous. Collectively they are referred to as plant pests. Three categories of plant pests can be differentiated. Insects such as caterpillars and leaf beetles chew on leaves and other plant organs (Fig. 8.19). They cause the most significant physical damage and tissue loss. Piercingsucking insects (e.g. thrips and mites) feed on plant cell content and cause comparatively little loss of plant biomass. Aphids and whiteflies are examples of phloem-feeding insects. They cause very little physical damage but can be dangerous to host plants as vectors for viruses.

Fig. 8.19. Insect herbivores. a Feeding tunnels of leaf miners (Photo: copyright obtained from Shutterstock). b Monarch (Danaus plexippus) caterpillar feeding on a leaf of milkweed (Asclepias syriaca). (Photo courtesy of the National Science Foundation, USA)
A second fundamental distinction, in addition to the feeding style, is that between generalist herbivores (which do not show much preference for certain plant species) and specialist herbivores (which seek particular groups of plants as food source). Specialisation is a consequence of the ability these insects have evolved to overcome the defences typical of individual plant families such as the Brassicaceae.
In spite of the abundance of herbivorous insects with their need to access the resources provided by photosynthesis, damage on plants is usually limited. Constitutive and inducible defences of plants act as effective barriers to the consumption of plant tissue by herbivores. While the discussion in this chapter focuses on insect herbivores, it should be noted that principally these defence mechanisms effectively restrict damage by vertebrate herbivores as well. Constitutive defences include morphological features such as thorns and trichomes. However, plant herbivore defence is predominantly chemical (Mithofer and Boland 2012). Low molecular weight compounds and proteins act as feeding deterrents or as toxins with direct inhibitory effects on insect growth and fitness. Molecules of chemical defence either are preformed and stored or are synthesised in response to the presence of feeding insects. Similar to pathogen defence, the inducible defence can be local and systemic.
An additional layer of herbivore defence is indirect defence (Fig. 8.20) (Heil 2008). Many plant secondary metabolites or their respective breakdown products are volatile and influence a plethora of organisms in the ecological communities around a plant. It is now well documented that organisms from other trophic levels can be attracted by plant volatiles. For instance, parasitoids of attacking specialist herbivores find their hosts with the help of plant-derived cues. Thus, the emission of volatiles results in a plant’s protection by the enemies of its enemies. A second mode of indirect defence relies on rewards (e.g. extrafloral nectar) provided by the plant for predators of herbivores.
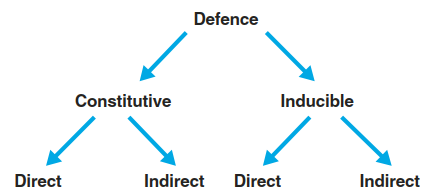
Fig. 8.20. Layers of herbivore defence
Constitutive Defences. Plants rely heavily on chemical defences against herbivores. As discussed in Sect. 8.2.1, plants produce a plethora of secondary metabolites. More than 200,000 different chemical structures have been identified and presumably many more exist in nature. Per plant species, several thousand of these metabolites can be expected. A major biological role is protection against herbivore attack.
The major compound classes implicated in herbivore defence are alkaloids, phenolic compounds, terpenoids (including steroids), cyanogenic glucosides and glucosinolates (Mithofer and Boland 2012) (Fig. 8.21). Their protective function can be due to toxicity, anti-digestive effects or the fact that they make a plant tissue unpalatable for herbivores. The modes of action or target sites in the herbivores, however, are barely understood at the molecular level.
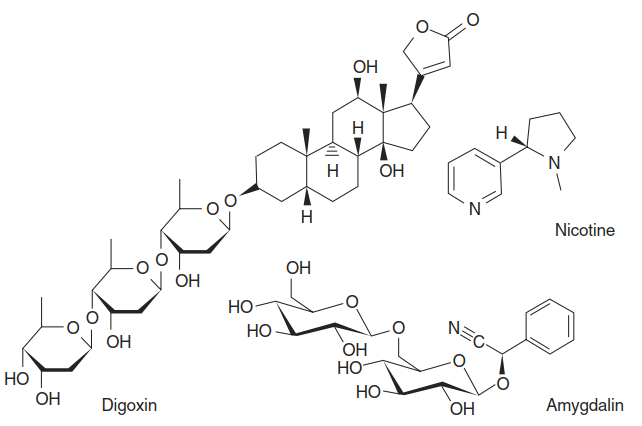
Fig. 8.21. Typical secondary metabolites with herbivore defence function. The steroid digoxin is a cardiac glycoside. It was isolated from the foxglove plant, Digitalis lanata, and is used as a treatment for various heart conditions because of its inhibitory effect on Na+-/K+-ATPases. Amygdalin is a cyanogenic glucoside that releases hydrogen cyanide. The alkaloid nicotine is synthesised in tobacco plants as a feeding deterrent and defence compound
The few existing examples illustrate principal mechanisms such as interference with neurotransmission, inhibition of metabolic processes or disruption of signal transduction pathways. A negative effect of toxic compounds on the producing plant—that is, self-intoxication—is usually prevented by storage in vacuoles. Furthermore, many defence compounds are stored in an inactive form. It is only after mechanical damage has caused contact with processing enzymes localised elsewhere in the cell or in different cells that the active compounds are released (see cyanogenic glucosides and glucosinolates below, Fig. 8.25).
About 20% of all plant species produce alkaloids—N-containing metabolites synthesised from various amino acids. Many alkaloids are known as potent toxins, effective against both arthropods and vertebrates. Modes of toxicity vary. Some alkaloids affect the nervous system. For example, caffeine (an alkaloid of Coffea arabica and many other plant species) can have a paralysing effect on insects by inhibiting phosphodiesterase activity at synapses. Another famous example of a toxic alkaloid with a defence function is nicotine (Fig. 8.21).
Nicotine is produced in the roots of Nicotiana tabacum and transported via the xylem to leaf cells, where it is stored in vacuoles. In insects it inhibits abundant postsynaptic receptors, the nicotinic acetylcholine receptors, thereby affecting control over muscle movement. Nicotine can, in addition, act as a deterrent. Using transgenic Nicotiana attenuata plants with blocked nicotine biosynthesis, it was shown that nicotine in floral nectar reduces nectar robbery and at the same time modulates the behaviour of pollinators in a way that increases reproductive success. Hummingbirds feeding on nicotine-containing nectar are discouraged from staying too long at any one flower by the unpleasant taste of nicotine. This results in more cross-pollination of tobacco plants and thereby promotes genetic diversity (Kessler et al. 2008).
Phenolic compounds (or phenolics) comprise a huge and chemically diverse group of metabolites sharing an aromatic ring with at least one hydroxyl group. They are found in all plant groups and have a wide range of functions—for example, as major constituents of plant cell walls (lignin, suberin), as flower colours (anthocyanins) or as ultraviolet protectants (flavones, flavonols). Many phenolics act as toxins or antifeedants. Tannins are synthesised by the majority of plant species. In the leaves of many woody dicots they accumulate to concentrations of up to 10% of dry weight (Barbehenn and Constabel 2011). Two major types are distinguished: hydrolysable tannins are polymers of simple phenolics and sugars and are localised mainly in cell walls; condensed tannins are formed by the polymerisation of flavonoids and are stored in vacuoles. Tannins can protect plants because they act as strong feeding deterrents for invertebrates and vertebrates. Beavers, for instance, prefer low-tannin poplars to high-tannin poplars.
The toxic effects of tannins are not understood in molecular detail and are thought to be due either to non-specific interaction with proteins or to pro-oxidant activities that result in oxidative stress in the gut of herbivorous insects. Overall, tannins can serve as an example of the lack of direct evidence as to the exact activity and effects of compounds widely implicated in chemical defence. In the absence of clearly defined genotypes that differ only in the molecules in question, it is difficult to infer the actual consequences of these molecules for the interaction of a plant with its herbivores. Such ecological research greatly benefits from genetic modification, as demonstrated by the nicotine example described above (Kessler et al. 2008).
Nonetheless, studies on tannins in poplar were among the first to illustrate concepts of community or ecosystem genetics (Whitham et al. 2006). For poplars differing in a quantitative trait locus (QTL) controlling total condensed tannin concentrations, it was found in common garden and field experiments that a variation in one trait (tannin concentration) has far-reaching consequences for the communities living around such a foundation species. For instance, the composition of arthropod communities and soil microbial communities is associated with the variation in condensed tannins controlled by this one QTL. Thus, one can refer to “community phenotypes” of individual tree genotypes. These phenotypes show considerable heritability (Whitham et al. 2008) (Fig. 8.22).
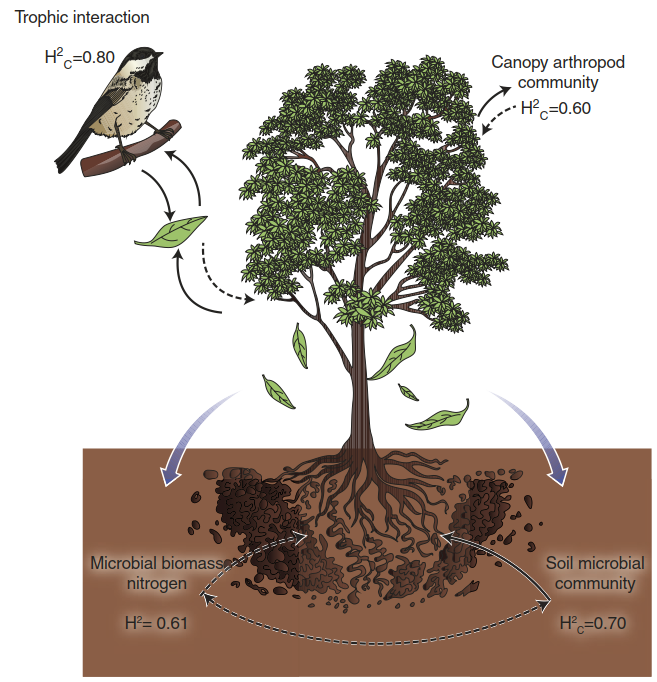
Fig. 8.22. Community phenotypes of poplar trees. The composition of communities living on and around a Populus angustifolia tree are strongly influenced by the genotype of the poplar individual, as determined in common garden experiments. This is apparent from the high degree of heritability (H2 and H2c)—that is, the large proportion of phenotypic variance attributable to genetic variance. One of the genetically determined variable traits is the content of hydrolysable tannins
Terpenoids represent another major class— with over 30,000 known structures in fact the largest class—of plant secondary metabolites. Terpenoids are derived from five carbon isoprene units. Besides the terpenoids that are essential players in plant biology as phytohormones (abscisic acid, gibberellins, brassinosteroids, cytokinins) or pigments (carotenoids), thousands of terpenoids are involved in plant-animal interactions, albeit with less defined functions for individual molecules. Terpenoids contribute to both direct and indirect defences. Many smaller terpenoids (monoterpenes, sesquiterpenes) such as linalool and α-farnesene are main components of plant volatiles.
Plant volatiles are often released upon herbivore attack, differ in their composition from the blend emitted before herbivory and can therefore attract parasitoids and predators of plant pests. Since the first reports on the attraction of parasitic wasps to maize plants emitting terpenoids in 1990 (Turlings et al. 1990) (Fig. 8.23), many more corresponding examples have been documented. Even the transfer of the ability to synthesise volatile terpenoids from one plant to another by transformation with a terpene synthase gene is sufficient to guide the choice of herbivore parasites (Fig. 8.24).
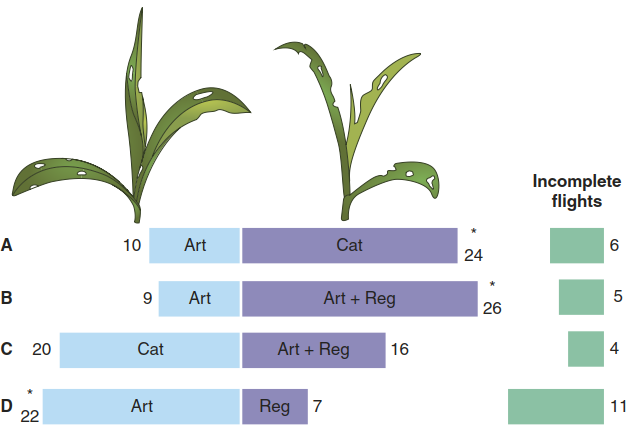
Fig. 8.23. Plant volatiles enable indirect herbivore defence. Choice experiments with parasitic wasps showed they prefer maize plants with caterpillar damage (Cat) over maize plants with artificial damage (Art) (A). Artificial damage treated with regurgitant (Art + Reg) (B, C) was nearly as attractive as caterpillar damage. Total numbers of flights are given with each bar. Asterisks indicate significant differences. (Turlings et al. 1990)
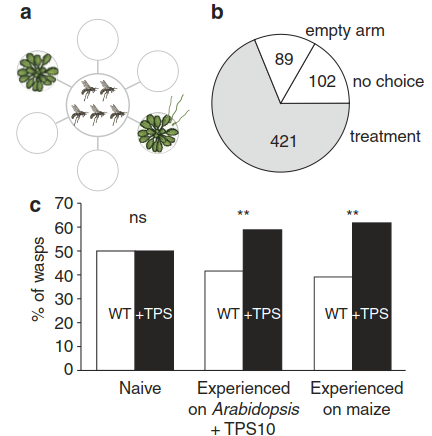
Fig. 8.24. Attraction of herbivore parasitoids by a volatile defence signal. Arabidopsis thaliana plants transformed with a terpene synthase (TPS10) produce volatile sesquiterpenes normally released by maize plants. Experienced individuals of the parasitic wasp Cotesia marginiventris prefer transgenic A. thaliana plants to wild-type plants. Transgenic TPS 10 sesquiterpene-releasing Arabidopsis and wild-type plants were placed in two arms of a six-arm olfactometer a to test the attraction of parasitoid females b. Three groups of parasitoids were tested c: naive wasps, wasps with a previous oviposition experience in a host larva in the presence of transgenic Arabidopsis emitting TPS10 sesquiterpenes, and wasps with a previous oviposition experience in a host larva feeding on maize. “Significant preference (p < 0.01) for the odour of the transgenic A. thaliana. (Schnee et al. 2006)
Direct defence is mediated, for instance, by the feeding deterrence of terpenoids in essential oils or resins, as well as the terpenoid volatiles emitted by some plants (Unsicker et al. 2009).
Steroids are triterpenes and thus are synthesised from isoprene units too. Among them are not only saponins (Fig. 8.6) but also cardenolides, the so-called cardiac glucosides. They inhibit Na+-/K+-ATPase and thereby interfere with maintenance of the membrane potential in neurons. Related molecules with a likewise known mode of toxicity are phytoecdysons. They mimic insect hormones such as ecdysone and thereby interfere with developmental processes in insects.
Hydrogen cyanide (HCN) is a classic example of a compound that can be toxic to the producing plants themselves and therefore has to be stored in a conjugated, inactive form. HCN affects mitochondrial respiration by inhibiting the binding of oxygen to cytochrome c oxidase and is thus a non-specific toxin. Many plant species (cassava, almonds, apricots and more than 2500 others) store cyanogenic glucosides in their vacuoles. When tissue is damaged by herbivore feeding, the glucosides come into contact with cytosolic glucosidases, the sugar moiety is cleaved off and cyanohydrin is released, which spontaneously or enzymatically is converted to HCN (Fig. 8.25).
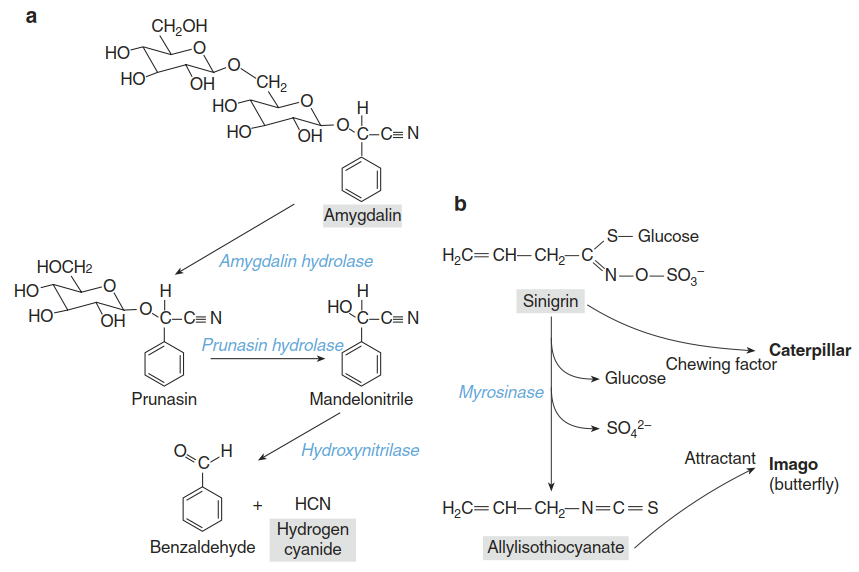
Fig. 8.25. Conjugated defence compounds and release of toxic compounds upon tissue damage. a The cyanogenic glucoside amygdalin occurs in seeds and leaves of many plant species. Upon wounding, the glucosidases amygdalin hydrolase and prunasin hydrolase become active. Following cleavage of the sugar moieties, hydroxynitrilases release hydrogen cyanide. b When cabbage leaves are damaged, the mustard oil glucoside sinigrin is released from the vacuole and comes into contact with a thioglucosidase (myrosinase). Removal of glucose by myrosinase results in a reactive intermediate, which is converted into volatile allyl isothiocyanate (allyl mustard oil). This compound, which has a pungent flavour to humans, is extremely toxic to many insects. For the specialist herbivore Pieris brassicae (the large cabbage white butterfly), the allyl mustard oil is, however, an attractant, which stimulates oviposition by the female butterfly
A second important class of conjugated defence compounds is the glucosinolates—sulphur- containing specialised metabolites typical of the order Capparales. Because Brassicaceae belong to this order and the model species A. thaliana belongs to the Brassicaceae, the mechanisms, evolution and diversity of glucosinolate biosynthesis are molecularly better understood than those of most other defence compounds. Glucosinolates are sometimes referred to as the “mustard oil bomb”. They are synthesised from various amino acids (e.g. methionine, alanine, phenylalanine, tyrosine and tryptophane) and stored in a glycosylated form in vacuoles. Upon tissue damage, glucosinolates come in contact with myrosinases—thioglucosidases that remove the sugar. Myrosinases are present only in a few scattered cells (Fig. 8.25).
The aglycones arising from the sugar cleavage are unstable and spontaneously rearrange into a variety of bioactive compounds, including isothiocyanates and nitriles. Isothiocyanates, in particular, are highly reactive and toxic to insects, mammals and many other herbivores. Volatile isothiocyanates are, in addition, known to attract organisms of the third trophic level (i.e. parasitoids of herbivores) and thus contribute to indirect defence (Hopkins et al. 2009). On the other hand, some herbivores specialising in Brassicaceae have evolved the ability to use isothiocyanates as cues for finding their host plant. These multiple roles of glucosinolates are major drivers of genetic diversity in the biosynthesis machinery.
Besides the amino acids used for protein biosynthesis, plants produce numerous non- proteinogenic amino acids as important primary metabolites (e.g. S-adenosylmethionine) or as secondary metabolites. Most of the latter are specific to particular plant species. Fabaceae tend to produce more non-common amino acids than other plant families. Among the functions of these metabolites is herbivore defence. The best-documented example is canavanine, an important nitrogen storage compound in the seeds of jack beans and other legume species. Canavanine is an arginine analogue, can be incorporated into proteins in place of arginine and is therefore highly toxic to organisms ranging from bacteria to man (Huang et al. 2011).
Constitutively synthesised defence compounds are found in all plant organs. Concentrations can vary strongly depending on the developmental stage, the environmental conditions and also the individual genotype (e.g. the tannin example discussed above). A substance particularly rich in defence compounds is latex, an emulsion exuded by about 10% of all plant species when mechanical damage occurs (Agrawal and Konno 2009). Probably the most famous example of a defence compound in latex is morphine in Papaver species.
In fact, many of the compound classes discussed above can be present at higher concentrations in latex than in leaves. This is why plant latex is sometimes compared with animal venoms, a complex mixture of low molecular weight and proteinaceous defence molecules, which exert a multitude of effects on a range of species. To date, few of the effects have been resolved molecularly. Mulberry leaves are toxic to insects other than the silk-producing Bombyx mori. Part of the toxicity can be attributed to alkaloids in the latex that resemble sugar molecules and thereby inhibit glucosidases. They are present in high concentrations (>1.5%; about 100-fold higher than in leaves) and are toxic to caterpillars (Konno et al. 2006).
An alternative to chemical defence has evolved in a limited number of plant species: hyperaccumulation of potentially toxic elements in leaves (= elemental defence). Selenium hyperaccumulation is the best-documented example; hyperaccumulation of Cd, Zn and Ni may serve similar functions (Cappa and Pilon-Smits 2013).
Date added: 2025-02-01; views: 347;
