Water Transport from the Soil to the Plant. The Second Part
In between these two contrasting types there is a group of moderately deep-rooting perennial plants, which use the water as it percolates through the soil profile. At times between these periods of precipitation, there are longer dry periods when these plants may even shed their leaves (i.e. they are deciduous). The differentiation of species according to their source of water applies not only to the Colorado Desert but also to other dry regions (e.g. the temperate semi-deserts in Argentina; Schulze et al. 1996b). Under arid conditions, water may also rise in the soil by capillary forces, but this will occur only above a water table. The hydraulic redistribution by plants is explained below (Fig. 10.11).
Water uptake by plants from the soil occurs in a zone behind the apex of the root tip where root hairs develop and the root cortex is not yet suberised (Steudle 1994, Steudle and Peterson 1998). In addition, water is taken up in meristematic regions of lateral roots. In older parts, the root is differentiated (Fig. 10.9a) into an epidermis below which a suberised layer called the exodermis exists in many species, followed by the root cortex, a heavily suberised or lignified endodermis (the Casparian band) and the central cylinder (the stele) with the xylem vessels and the phloem. Water follows (as a first approximation) the water potential gradients from the soil to the xylem, but along various pathways (Fig. 10.9b).
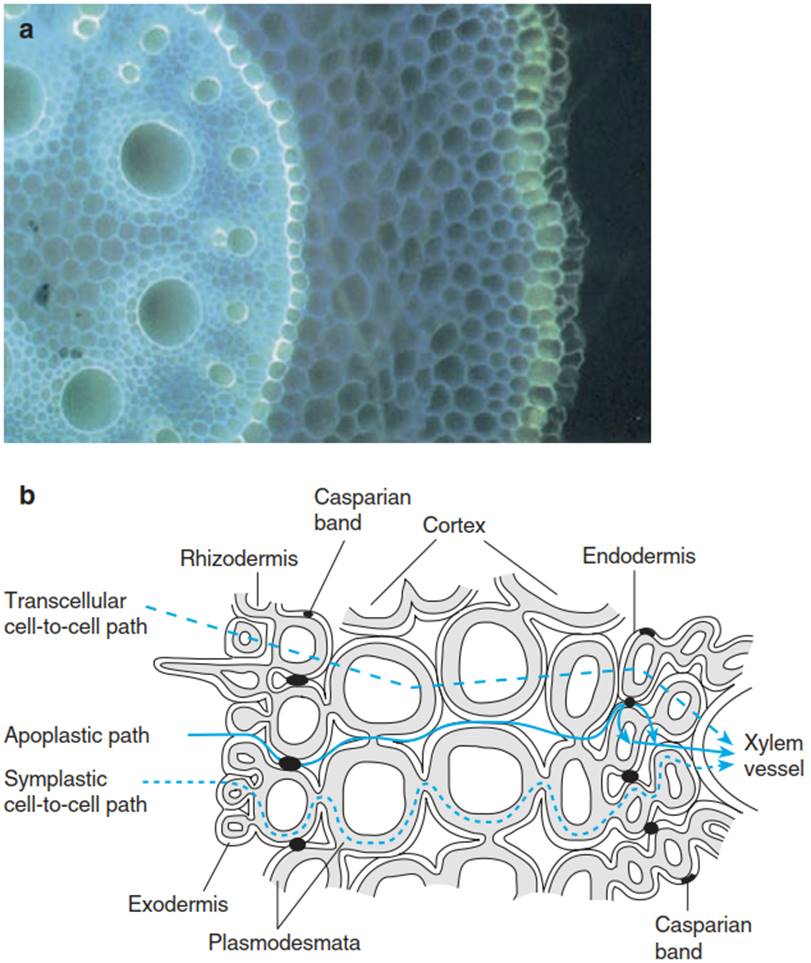
Fig. 10.9. Water flow through the root. a Cross-section through a maize root in which the lignin and lipids in the exodermis and endodermis are stained with berberin sulphate. b Schematic crosssection of a root showing routes of water and nutrient transport. The suberised Casparian bands appear as black dots in this crosssection, showing their position in the cell wall. Blue arrows mark clearly different paths that water can take. (After Steudle (1994))
In the region of the root cortex, water may (a) flow in the cell wall (apoplast), (b) move from cell to cell via the plasmodesmata (symplast) or (c) move across the cells (via the transcellular path). At the endodermis (and probably also at the exodermis), water must be moved through the symplast of specialised unsuberised transmission cells. In the undifferentiated root tip, where the endodermis does not yet exist, water may enter via the apoplast and the symplast of growing cells. Thus, water transport in the root may be described by a model with a series of parallel resistances connected by serial resistors. The hydraulic conductivity in each cell layer (Lpz, measured in metres per second per Megapascal; Eq. 10.12) may be described by:
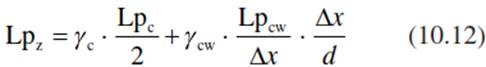
where Lpc describes the hydraulic conductivity of cellular membranes and the factor 2 considers the fact that two membranes per cell must be crossed. Lpcw is the hydraulic conductivity of the cell wall, γc and γcw are the amounts of cytosol and cell wall at the cross-section in the direction of the flux (γcw + γc = 1), ∆х is the width of a cell layer and d is the thickness of the tissue. Equation 10.12 shows that the relevant fluxes may be distributed differently according to the structure of the root. The distribution of the pore size in the cell wall and the size of the hydrated ions determine the conductivity. Pores in the inter-micellar space of the cell wall are about 5 nm; the space between the cellulose fibrils is about 10 nm. In comparison, a water molecule diameter is about 0.3 nm, Na ions with their hydrated shell reach 0.5-0.7 nm, K ions measure 0.4-0.5 nm and a glucose molecule measures about 0.75 nm (Luttge and Higinbotham 1979). Even though the cross-section available for apoplas- tic transport is much smaller than that for the cytosol, it depends on the conditions if the flux is hydraulic (following the water potential gradient of transpiration) or metabolically regulated following an osmotic gradient. Thus, the root is not a uniform structure (Steudle and Peterson 1998).
If the apoplast path is interrupted (e.g. by strong suberisation of roots), the cellular component dominates (Michael et al. 1997). The flow of water through the cell membrane in the cellular transport path is affected by aquaporins (proteins that act as valves in the hydrophobic membrane of the cell) (Tyerman et al. 1999) (Chap. 6).
Even though water transport through the endodermis is metabolically regulated, water flow can be inhibited by only about 50% by mercury, which binds to sulphur-containing amino acids in the aquaporins. Also, antisense-plants lacking aquaporins maintain 40% of the hydraulic conductivity of control plants (North and Peterson 2005).
The water transport across the Casparian band results in a positive pressure, which is visible on cut stems as emerging droplets. The root pressure is caused by an osmotic gradient of about 100 mM of inorganic ions, typically under conditions with high moisture or low/no transpiration. This results in a pressure in the order of 1-2.5 MPa and may lift water from the root to the leaves up to a height of 25 m (Nobel 2009; Lambers et al. 1998). However, for water transport in dry air, this process is not sufficient to carry enough water; it may however be important in healing cavitation of xylem elements (Fig. 10.15).
The differentiation of root anatomy is, from an ecological point of view, a response to the conditions of water flow between the soil and root, and the uptake of nutrients. Water uptake normally does not limit the supply of water to the plant in moist soils. The water potential in the shoot is, of course, also determined by transpiration. Thus, in moist soils, the water potential in the xylem decreases (becomes more negative) with the amount of water that is transported through the system (Fig. 10.10, curve A), the slope representing the hydrologic resistance (gradient/flux) or hydraulic conductance (flux/ gradient) of the root and the stem. In ecology, “conductance” is the preferred variable because it is proportional to the flux (Cowan 1977).
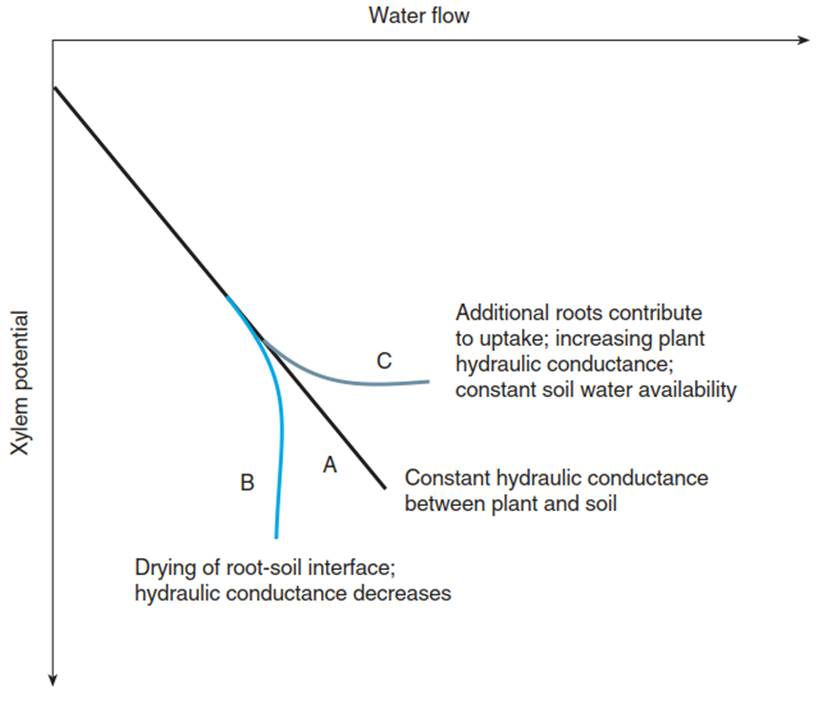
Fig. 10.10. Schematic diagram of changes in xylem water potential with increasing water flow. A The hydraulic conductance is constant. B The hydraulic conductance falls when the interface between root and soil dries out. C Additional roots contribute to the transport as the soil dries
In dry soils, corresponding to the low flux and the very low conductivity in the unsaturated soil, a dry zone may develop around the surface of the root—that is, further supply from the soil may, in this case, be the limiting factor (Michael et al. 1999). This state would be visible in the leaf by a strong reduction of water potential in the xylem without an associated increase in the flux through the vessels (Fig. 10.10, curve B). A corresponding turgor loss is expected to occur in the root tip under such conditions, leading either to osmotic adjustment or to production of the stress hormone abscisic acid (ABA) (Chap. 6).
Conversely, there are situations where changes in water transport may occur without changes in water potential (Fig. 10.10, curve C), —for example, by water availability in specific horizons. Here, the water potential gradient between the soil and xylem reaches a magnitude that allows a flux via additional surfaces (i.e. other roots or root regions) that were not participating in water uptake because the potential gradient was too low. Thus, the hydraulic conductance of the root, and thus water uptake, is variable, and it is plant regulated through the root architecture (Ewers et al. 2000) and the molecular responses to drought in the root tip.
The site for water uptake also harbours a potential leak. The plant cannot seal itself against the soil. “Unprotected” regions are the root tips and the axial meristems of lateral roots (i.e. the regions of water uptake into the roots). Also, the transmission cells in the endodermis and in the exodermis may transport water in both directions—that is, roots not only are able to take up water from the soil but also may lose water to the soil. This is ecologically important at low transpiration. During the hydraulic lift (also called hydraulic redistribution), water is taken up from wet soil (often in deep horizons) and moved by a water potential gradient to another (mainly the upper) soil horizon, where it is released into the dry soil (see reviews by Neumann and Cardon (2012) and Prieto et al. (2012)). Water release of plants to the soil was first observed in dry climates (Richards and Caldwell 1987), but it is also important in temperate climates. For Acer saccharum it was observed (Dawson 1993) that the isotopic composition of soil water in the region of the canopy corresponds not to the rainwater but to the much deeper groundwater (Fig. 10.11).
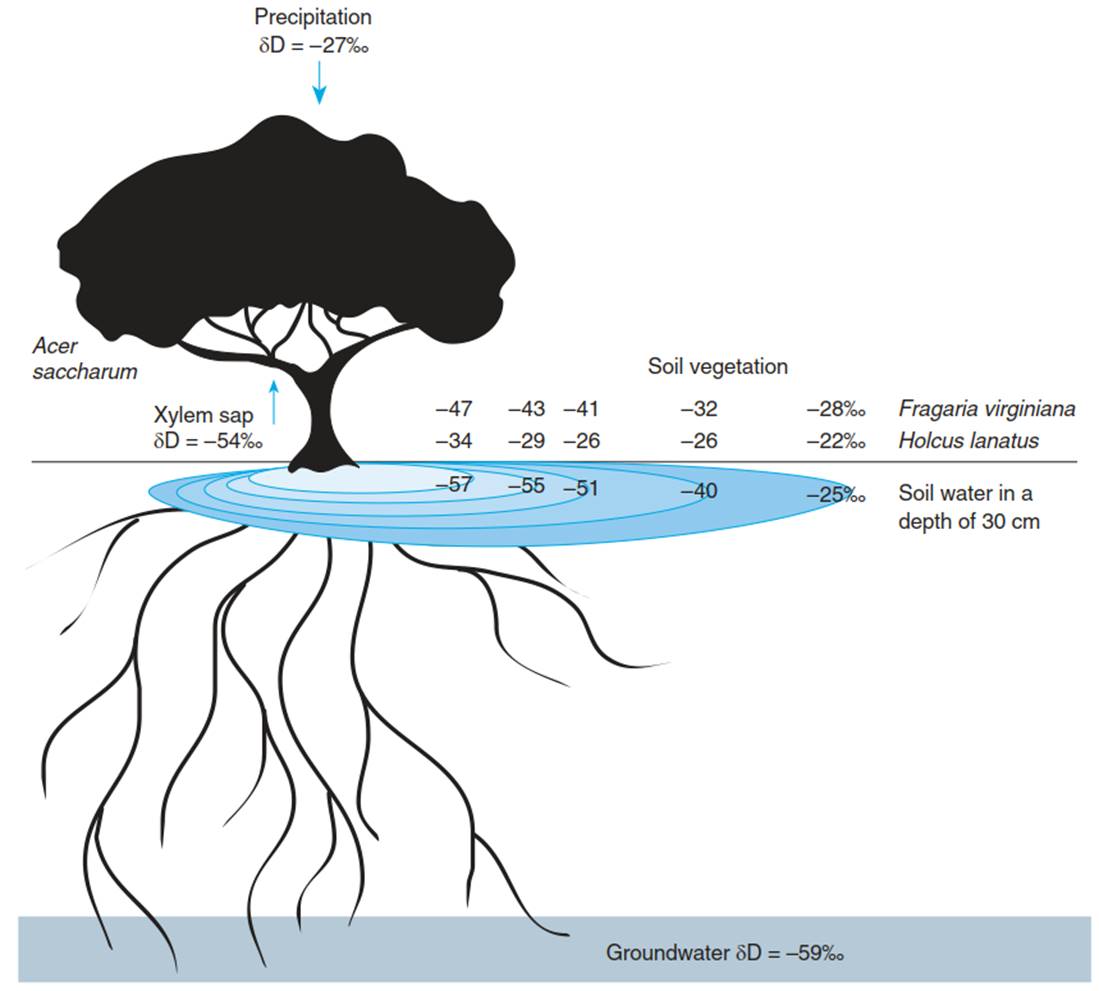
Fig. 10.11. Stable isotope values of hydrogen (δD values) as a marker of the source of water. The δD value is calculated from the D/H ratios of the sample in comparison with a standard [(D/H)sample/(D/H)standard - 1)], where water from deep oceans is used as the standard (V- SMOW = Vienna standard mean ocean water). The δD value in vegetation is between the high value in rainfall and a significantly lower δD value in the groundwater. The lower δD value in the xylem water shows that Acer saccharum derives its water from the groundwater.
The soil water (0-30 cm deep) shows a gradient in the δD value, from the low value near the trunk to a high δD value away from the trunk. The water with the lowest δD value can only have come from groundwater that is transported by the roots and is released into the soil, during the so-called hydraulic lift. The vegetation reacts differently to the water availability depending on how deeply the roots penetrate. While Fragaria virginiana is able to use the “lifted” water, the roots of Holcus lanatus do not go that deep. (After Dawson (1993))
The smaller the distance to the root of the tree is, the more similar the isotopic composition of the xylem water of the herbaceous vegetation is to that of the tree xylem water. Obviously, during the night, larger amounts of water are transported by tree roots from the moist subsoil above the water table into the dryer topsoil. The isotopic signature of the water of the topsoil changes correspondingly. The water is utilised during the day not only by the tree but also by the vegetation covering the ground in the shade of this tree (“water parasitism”). In this example, 30-60% of the water in the xylem of this ground flora originates from the hydraulic lift of the tree roots. This example may explain the often luxurious vegetation of herbaceous species in the shadow of trees in semi-arid regions.
The reverse process, the inverse hydraulic lift (Schulze et al. 1998a; Burgess et al. 2001), is ecologically just as important as the hydraulic lift. In arid regions the lower soil layers may very rarely be moistened as precipitation is sufficient only to wet the upper soil layers to field capacity. The “wave of water” produced in the soil by precipitation penetrates only a few decimetres (in silt) or metres (in sand). Lower soil layers remain permanently dry unless there is groundwater. Nevertheless, roots are able to penetrate such dry soil to a considerable depth by the inverse hydraulic lift (Fig. 10.12; Canadell et al. 1996).
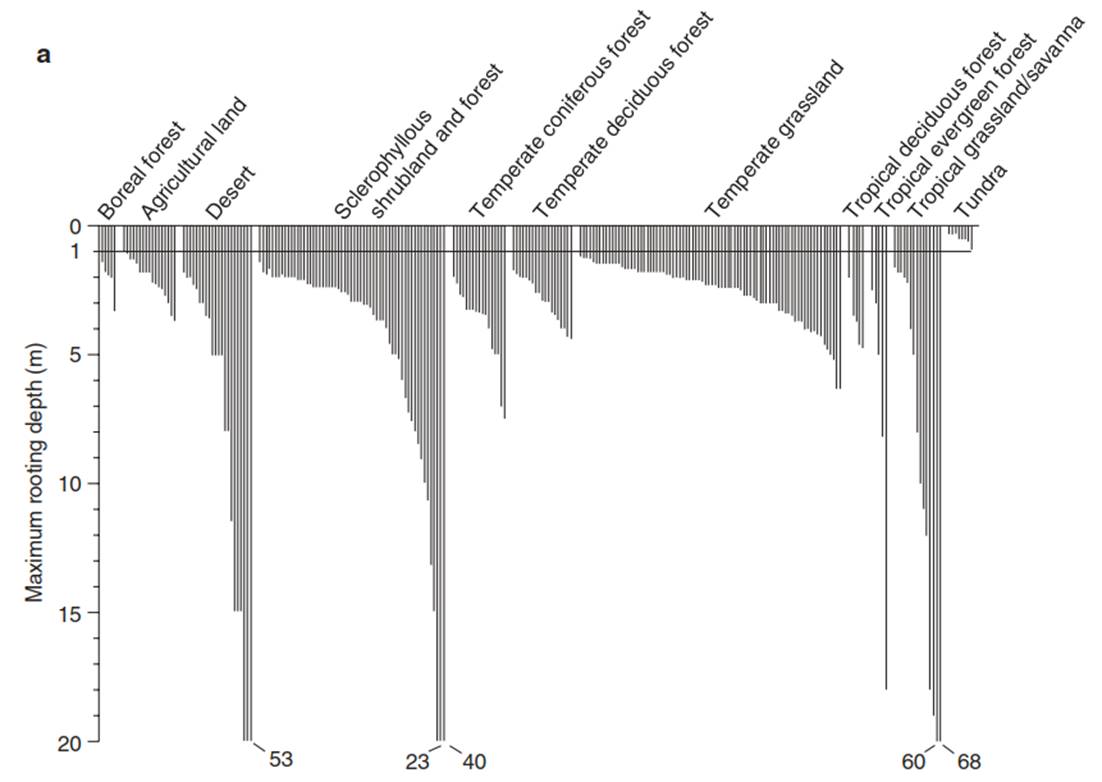

Fig. 10.12. Depth of roots in global vegetation types. a Maximum observed rooting depth of different types of vegetation. Each line represents a single measurement of a particular plant species. The numbers show the maximum depths that are beyond the y scale (from Canadell et al. (1996)). The deepest known root depth was measured in the Kalahari Desert from Acacia erioloba when bore holes were sunk to the water table. b Acacia erioloba savanna with perennial C4 grasses Aristida and Stipagrostis (see Schulze et al. (1996a)) in the Kalahari Desert, north of Uppington, South Africa
The maximum rooting depth in deserts and savannas is more than 50 m. The absolute record for observed root depth is 68 m in the Kalahari, where the groundwater is more than 100 m deep, covered by dry sand, and it is expected that roots are able to penetrate to that depth. Roots up to 100 m deep have not yet been found. The deepest roots were found by chance during construction of a well. Penetration of roots to such a depth in dry soil is possible only by transport of water from the moist topsoil (i.e. the inverse hydraulic lift)—that is, the root must be kept wet in a very dry rhizosphere. Without additional transport of water, the root tip, which is protected only by mucilage, would desiccate in the dry soil.
Even though water uptake occurs predominantly in roots, liquid water may be taken up by shoots even from fog and dew via lenticels of the bark (Klemm 1989) and via water films of the stomata (Burkhardt 2010).
Date added: 2025-02-05; views: 520;
