Xylem Water Transport
A stem structure distinction is made between herbaceous and woody species, but many transitions exist. In fact, the xylem elements of all herbaceous species contain lignin to achieve the needed rigidity of vessels, which operate under tension. Thus, Schweingruber and Buntgen (2013) concluded that the classification between woody and herbaceous species is not supported by wood anatomy. One may also distinguish between stems according to the structure of the vascular systems. In closed vascular bundles, the initial meristem between xylem and phloem cells, the cambium, terminates cell division. The xylem and phloem are surrounded by a vascular bundle, the bundle sheath, most conspicuously developed in grasses (Fig. 10.9).
In contrast, open vascular bundles exist in most dicots where the cambium remains active and produces xylem cells towards the plant interior and phloem cells towards the outer periphery of the stem. An example is the herbaceous species Arabidopsis thaliana, which contains a stem anatomy identical to that of woody stems (Fig. 10.13a). Other herbaceous species (e.g. Polygala alpestris) even exhibit seasonal growth rings (Schweingruber et al. 2013). In trees and shrubs, a distinction is made according to the arrangement and size of vessels during the course of the growing season. In diffuse porous wood (Fig. 10.13b), vessels and tracheids of different diameter are formed according to the demand of water flow at any time of the season (e.g. in Betula pendula). The wood is very similar to that of Arabidopsis thaliana, except that large vessels exist only in trees and shrubs. In ring-porous wood, a ring of very large vessels is formed when growth is initiated after winter and new leaves develop (Quercus robur; Fig. 10.13c). With ongoing seasons, smaller vessels and even tracheids follow.
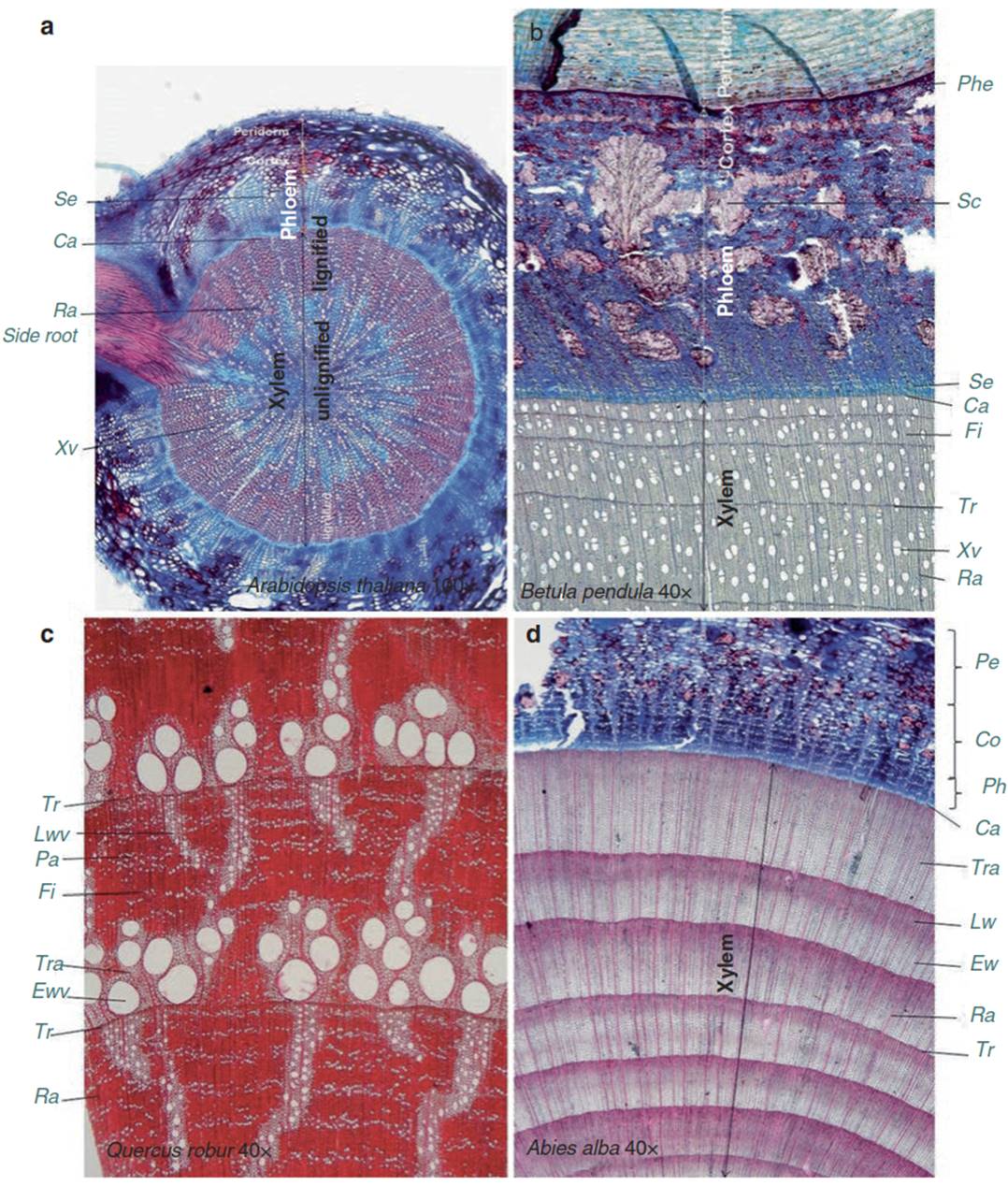
Fig. 10.13. Wood anatomy of plant types. Cross-sections of a the herbaceous stem of Arabidopsis thaliana with secondary xylem cells, b the diffuse porous wood stem of Betula pendula and c the ring-porous Quercus robur. d Coniferous wood of Abies alba. Ca cambium, Co cortex, Ew early wood, Fi fibre, Lw late wood, Lwv late wood vessel, Pa parenchyma, Pe phellogen, Ped periderm, Ph phloem, Ra ray, Sc sclerid cell, Se sieve elements, Tr tree ring, Tra tracheids, Wwv early wood vessel, Xe xylem element, Xv xylem vessel. (Anatomical sections by F. Schweingruber)
In all cases the xylem elements are formed by an open meristem where the cells die after elongation. The cambial activity is needed in long-lived species because phloem elements are relatively short lived. Since cell division of the cambium results in phloem and xylem elements, the dead xylem cells accumulate and remain functional for water transport, connecting the living root central cylinder with the living leaf mesophyll. The size of the vessels is determined by a plant-hormonal balance in the cambial layer (see Schweingruber et al. (2013)). Since there is also a balance between the leaf area index (LAI) of transpiring leaves and the total xylem area that transports water, the long-lived xylem elements become dysfunctional if they do not participate any more in water transport. Under these conditions, the remaining meristematic cells of the wood seal the xylem elements mainly with tannins to make these elements resistant against fungal attack.
Thus, we distinguish between an outer ring of xylem elements (the sap- wood), which participates in the water flow, and an older inner part of the wood (the heartwood), which is not conducting water but stabilises the stem (Fig. 10.14). Because of the tannins, this wood is generally darker-coloured, but there are species where the heartwood is not clearly visible (e.g. Picea abies). It is the heartwood that gives a species the physical strength for larger structures. With heartwood formation, most meristematic cells also die, but some meristematic cells may remain alive for more than 100 years (in Carpinus betulus) (Fritzsche 1910). It should be noted that young trees contain only sapwood. Heartwood formation occurs when the stem area increases beyond a required sapwood area. Thus, the number of conducting elements in woody plants is regulated by the annual increase of new elements as well as by the transition of old elements from sapwood to heartwood (Fukuda 1997). Only the sapwood area conducts water.
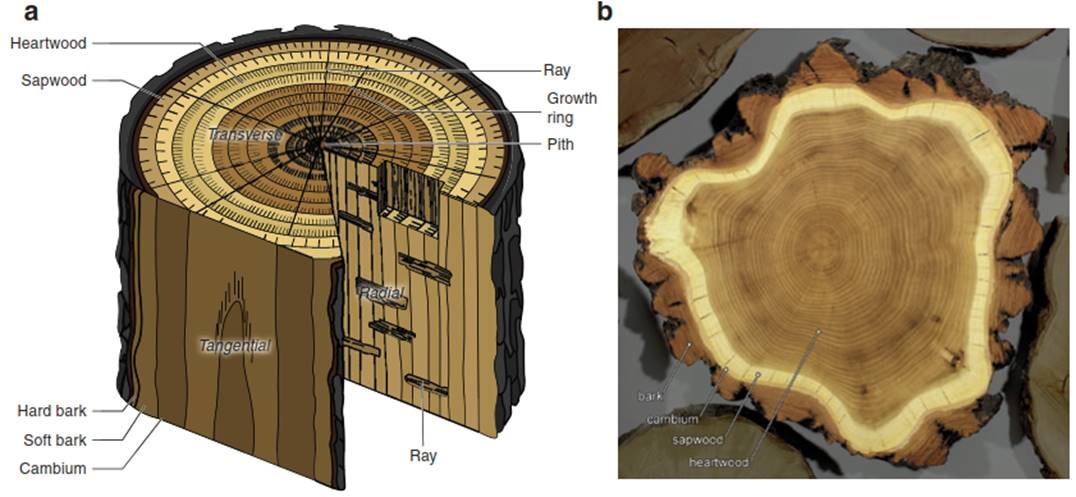
Fig. 10.14. Wood anatomy. a Section of a piece of wood with its main structures. b Cross-section of Robinia pseudacacia, with light-coloured sapwood and dark-coloured heartwood
The cambial activity is not constant but highly dependent on the growing conditions. Cambial activity ceases only during winter and during dry periods. This change in cambial activity results in growth rings (called tree rings in shrubs and trees) of variable activity. Since the anatomy of wood does not change any more after cambial division and elongation, tree rings are a “fossil” record of the growing conditions in the past and have been used to reconstruct the climate, on the basis of statistical models, explaining present-day ring width with climate conditions during the growing season (Vaganov et al. 2006). There are species that do not form a ring structure but terminate growth under conditions of drought without an obvious anatomical signal. Trees in humid tropical regions without any dry season also have no annual tree ring structure but exhibit visible changes in growth activity.
Xylem water transport follows the water potential gradient between the root and leaf during the day. The hydraulic conductivity of the xylem is relatively high. The question of the physical conditions in a capillary with negative pressure of more than 10 MPa has been a topic of research for many years. Bohm (1893) was probably the first to postulate that the cohesion between water molecules is sufficient to achieve a continuous water column in the xylem vessels under tension (cohesion theory). With the measurement of negative pressures of more than 1 MPa in xylem vessels (Wei et al. 1999) and the observation that the tension changes with the flux through the xylem vessels, the cohesion theory has also been confirmed by measurements.
Biophysically the water flux in a xylem vessel, Jx (in cubic metres per second), is described by the Hagen-Poiseuille law for laminar flows and depends on the radius, r, of the xylem vessel, the viscosity of the liquid (ⴄ = 10-3 Pa s for water) and the hydrostatic gradient, dP/dx (in Pascals per metre):
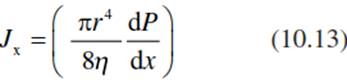
The flow is in the direction of the decreasing hydrostatic or water potential gradient (from less negative to more negative values). The flow must be sufficiently slow so that the conditions of laminar flow (in contrast to turbulent flow) are maintained, to avoid rupture of the water columns. The potential gradient required to transport a certain volume flow (e.g. 1 mm s-1) across the cell wall is very high, about 3 x 105 MPa m-1, according to Eq. 10.13 (Nobel 2009). Thus, major forces are required to move water through the cell wall of the leaf mesophyll, which leads to relatively slow movement of water in the xylem.
In addition to the regulation via the vessel diameter, the volume flux per time and unit area, Ix (measured in metres per second), in the xylem under a pressure gradient (∆Р/∆х) is determined by the area of the cross-section per vessel and the number of xylem vessels, n, per organ:
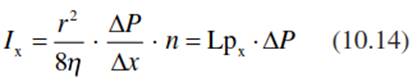
where Ix is measured in (square metres x Pascals)/(seconds x Pascals x metres) = (metre per second) and Lpx corresponds to the hydraulic conductivity in the xylem (measured in metres per second per Pascal). The axial hydraulic conductivity is related to a 1 m length of xylem and thus has different dimensions from the Lp of the membrane.
Following Eqs. 10.13 and 10.14, plants have many possibilities to regulate the flux in the xylem and thus the water potential gradient, or the water potential gradient and the concomitant flux (Gartner 1995).
The vessel radius varies between 500 pm in lianas to approximately 100 pm in ring-porous woody plants (e.g. oak) and 10-40 pm in the tracheids of conifers (Table 10.2); a larger radius allows a considerably higher volume flux. In tropical lianas, in ring-porous woody plants and in dicotyledonous herbaceous plants, water taken up by the root reaches the transpiring leaf in less than 1 h. In contrast, it takes 2-3 months for the water taken up by the roots to reach the tip of a 100 m high Sequoia gigantea, because of the low average rate of flux during only part of the day.
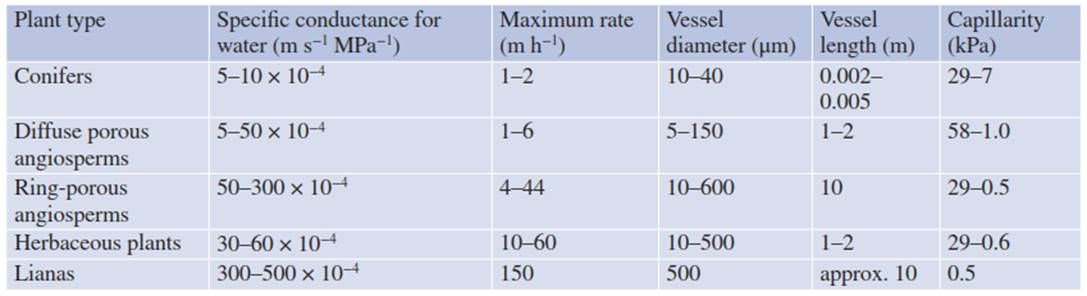
Table 10.2. Anatomy, conductance and water flow in the xylem. Vessel radius and length, and maximum capillarity of vessels from different types of plants according to Zimmermann (1983), Carlquist (1991) and Nobel (2009)
At a constant volume flow, the water potential decreases (i.e. gets more negative) with increasing radius of the vessels. However, the advantage of wide vessels for rapid transport of large amounts of water is counteracted by the increased risk of cavitation (Tyree and Sperry 1989). The forces of cohesion, maintaining a continuous column of water in the xylem, decrease with increasing radius of the xylem vessels (Eq. 10.13) from about 1500 Pa in tracheids of conifers (r = 10-40 µm) to about 60 Pa in tracheids of ring- porous woods (r = 500 µm; Table 10.2).
Thus, there is the danger that the cohesive force determining the continuity of the water column is exceeded. Cavitation describes the breakage of the water column in xylem cells. It is caused by small air bubbles, filled mainly with water vapour, forming in a thermodynamically unstable condition (Tyree 1997; Steudle 2000, 2001; Stroock et al. 2014). Once initiated, the bubble expands, causing an embolism in the xylem cell—for example, after injury. During the breakage of the water column, the flux in the vessel is interrupted. In wood, the pits of cell walls that separate xylem vessels seal cavitated vessels off. The water transport is redirected laterally (Grace 1993). Cavitation may be healed by various processes (Holbrook and Zwieniecki 2005). At high water potential, water vapour may condense again, restoring water column continuity. Cavitation and embolism may also be healed via root pressure if the plant is not too tall (Sperry et al. 1987; Gartner 1995). Plants may also be able to refill cavitated xylem vessels by phloem water because of the difference in the water potential in adjacent parenchyma (Holtta et al. 2006; Nardini et al. 2011). However, if cavitation affects complete organs (leaves or branches), these parts dry and die.
Since the risk of cavitation increases with increasing size of xylem vessels (Fig. 10.15a) (Lo Gullo and Salleo 1991), cavitation occurs first in vessels with a large lumen, while the water column in vessels with a small lumen remains intact, even at high water tension. The structure of the conducting tissue determines the risk of cavitation at high rates of water transport into the shoot (Grace 1993). However, the plant is not unprotected in the face of this danger. With increasing drought, water transport in the soil and root changes, but the stomata will restrict the water flow (Sect. 10.3), and the relation of the leaf area to the xylem conducting area can be regulated by slowing of leaf formation or by shedding of leaves. Loss of productivity and plant mortality have been explained by hydraulic failure (Choat et al. 2012).
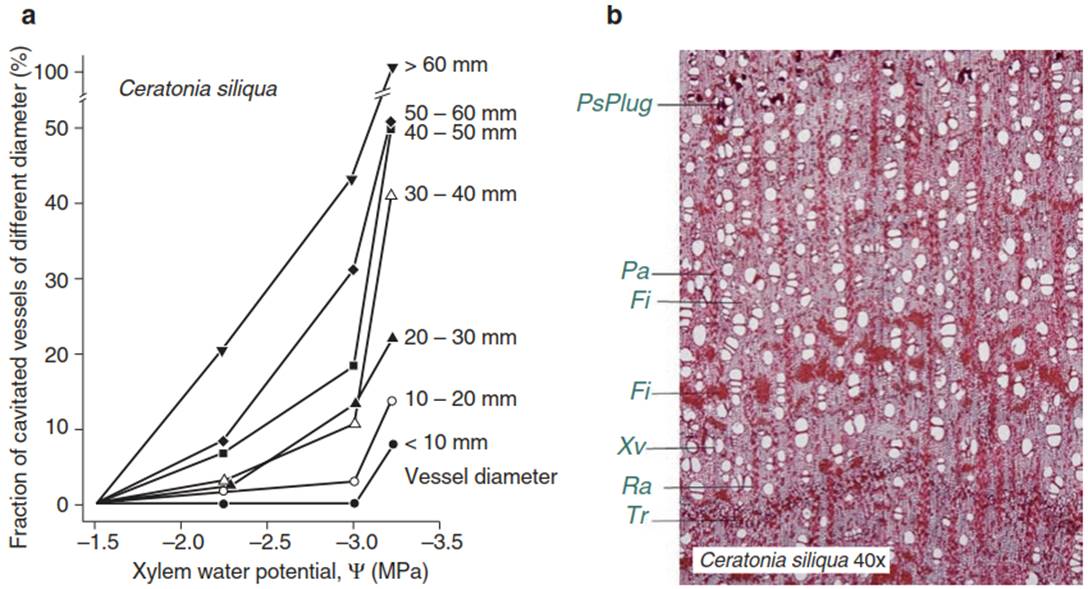
Fig. 10.15. Cavitation in xylem vessels. a Dependence of cavitation in xylem vessels of different size on the water potential in the xylem as the soil dries over several days. At a water potential of approximately -3.3 MPa, all large vessels of Ceratonia siliqua (>60 mm), but only 10% of the small vessels (<10 mm), are cavitated (Lo Gullo and Salleo 1991). b Cross-section of C. siliqua, consisting mainly of thick-walled fibres. Vessels with variable lumina are arranged in short radial rows. Large vessels in the older part of the wood are closed by thylosis, which are formed by cavitation during drought, whereby vessels that no longer participate in the water flow are sealed. The border of a growth ring is hardly visible, as indicated in the maritime climate of the island of Cyprus, where the investigated stem of Ceratonia grew. Fi fibre, Pa parenchyma, PsPlug phenolic substance plug, Ra ray, Tr tree ring boundary, Xv xylem vessel. (anatomical section by F. Schweingruber)
Obviously, species “adapted” to a habitat have generally evolved mechanisms to avoid lethal stress situations. Other species would not flourish in these habitats or would restrict their growth and reproductive phase to a short period in which this critical situation does not occur. For example, the Mediterranean Bromus spp. are successful invasive species in North American prairies and Australian semi-deserts, where the vegetative growth is restricted to the period with sufficient water supply. The invasive Bromus spp. gain this water with shallow roots from the top layers of soil at the cost of the water supply to indigenous perennial dwarf shrubs, particularly Artemisia tridentata in North America and Atriplex spp. in Australia (West and Young 2000) (Chaps. 13 and 20), which have deep-reaching root systems.
Generally, the diameter of vessels is larger in roots than in stems of the same species (Martinez-Vilalta et al. 2002), further decreases in peripheral organs like branches and twigs, and is particularly small in the petioles of leaves. While water potentials in the xylem decrease with increasing distance to the soil within the water conduits, the danger of cavitation is increasing. However, the small vessel diameters in the petioles reduce the risk of progressive cavitation.
At constant hydraulic conductivity, the water potential in the leaf decreases linearly with increasing transpiration. This can be used to demonstrate structural differences in the stem between species (Fig. 10.16a) (Schulze and Chapin 1987). Plants with lower xylem conductivity (i.e. with a steeper slope in volume per time and unit area and water potential gradient) have lower rates of transpiration and more negative water potentials. In contrast, plants with high xylem conductivity (i.e. with a flatter slope) also have high rates of transpiration; however, the water potential does not decrease to the same extent.
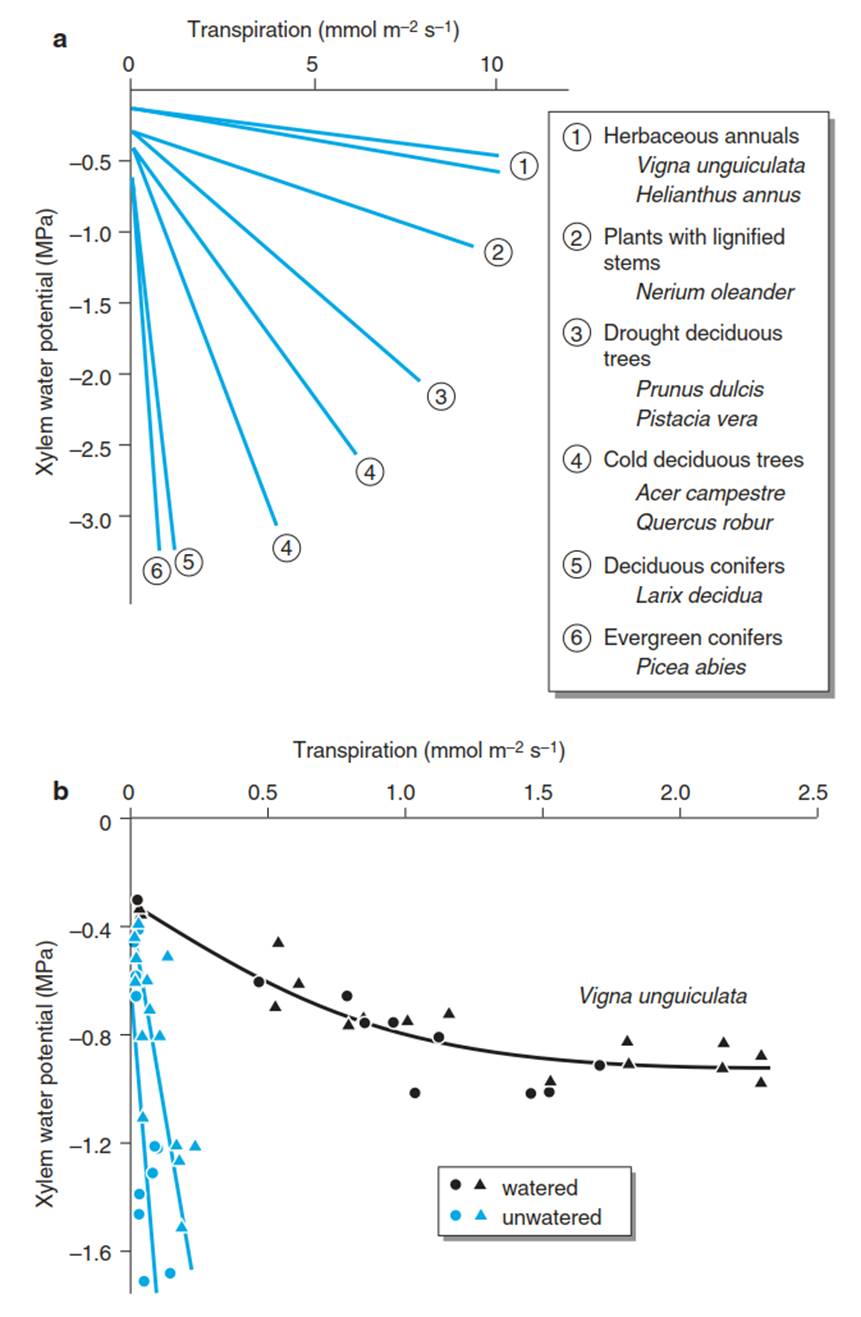
Fig. 10.16. Relationships between water potential in the xylem and transpiration a for different plant functional types (Schulze and Chapin 1987) and b for the crop plant Vigna unguiculata in drying soil (Schulze and Hah 1982). An increasing transpiration rate causes a lowering of the water potential. The slope of the graph is a measure of the hydraulic conductivity of the plant-soil system. Circles and triangles show the two groups of plants measured. (see also Fig. 10.10)
In these species, the risk of cavitation is particularly great, leading to a substantial change in conductivity when the soil dries out (Fig. 10.16b) (Schulze and Hall 1982). In these latter species, the xylem water transport under moist conditions takes place in vessels with large lumina, while under dry conditions, xylem water transport is restricted to vessels with narrow lumina, which cavitate rather late (Fig. 10.15b). Regulation of water flux via structural characteristics of the shoot is thus possible and is dependent, to some degree, on the conditions under which a species grows.
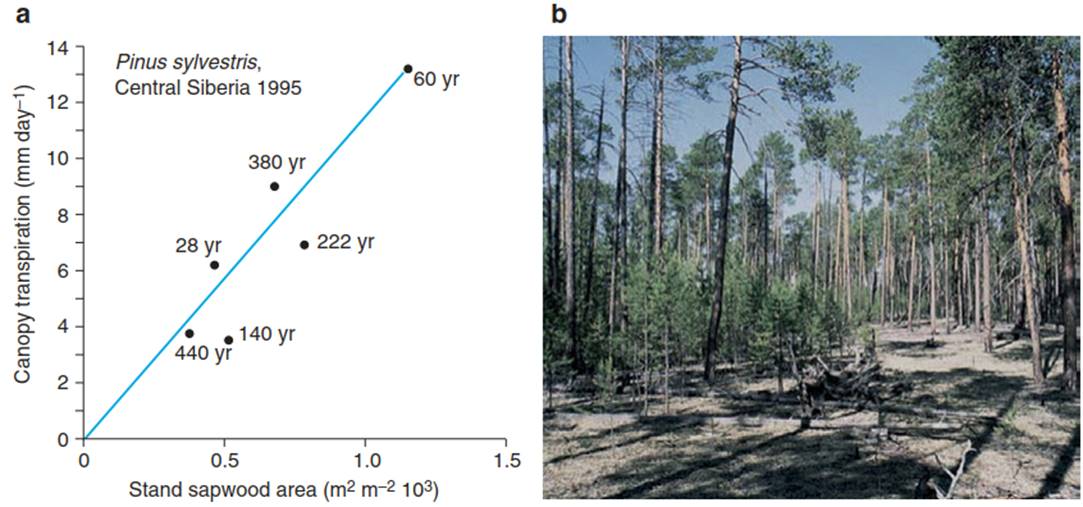
Fig. 10.17 Relation between canopy transpiration and stand sapwood area. a The rate of water flow rises Linearly with the sapwood area; note that it is neither the youngest nor the oldest and largest trees that have the highest transpiration rates, but the 60-year-old pole stand. The small area of sapwood in the oldest stand is caused mainly by the low tree density in older stands (Modified from Zimmermann et al. (2000)). b Pinus sylvestris woods on Pleistocene sand dunes in central Siberia at 60°N on the west bank of the Jennesey. Here, cohorts of even-aged trees establish after fire (see Wirth et al. (1999)). The vegetation of the forest floor is Cladonia
The role of the xylem structure in water transport is demonstrated by measurements of water flow in the xylem of pines of different ages in the continental climate of central Siberia (Fig. 10.17) (Zimmermann et al. 2000), where the xylem flow increases linearly with the sapwood area. The greatest sapwood area is achieved at the age of 60 years. At this age the growth rate of trees is relatively high and the formation of heartwood has not yet started. In very old pines the sapwood area decreases, and thus the flow of water decreases. A certain plasticity in xylem development during radial growth according to the water supply has also been described (Eilmann et al. 2010; Plavcova and Hacke 2012; Bryukhanova and Fonti 2013).
Date added: 2025-02-05; views: 610;
