Mineral Nutrients
An element is essential when an organism cannot complete its life cycle without that element. Depending on the concentration found in tissues, mineral nutrients are divided into macroelements (approximately 1000-15,000 μg/g of dry weight (d.w.)) and microelements (approximately 0.005-100 μg/g d.w.) (Table 7.1). A third category comprises the beneficial elements that have positive effects on plant growth and/or fitness but are not essential.
Silicon is accumulated by many plant species, sometimes to very high levels, and accounts for up to 10% of the dry weight of leaves of grass species such as Oryza sativa. Silicon accumulation protects against several abiotic and biotic stresses because deposition in the cell walls strengthens the physical barrier against invading pathogens, enhances tolerance of mechanical stresses and reduces cuticular transpiration (Ma and Yamaji 2006). Still, there is no evidence that a plant cannot exist without this element.
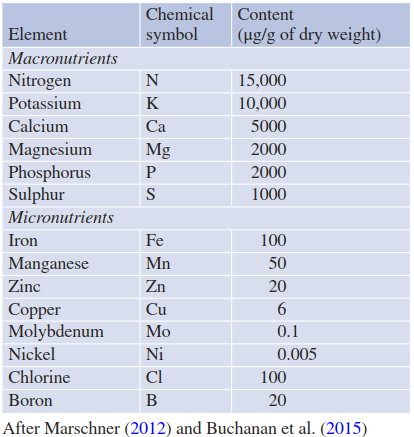
Table 7.1. Adequate concentrations of macronutrients and micronutrients
Generally not counted as nutrient elements are H, C and O, which are by far the most abundant elements in organisms. They are obtained not from the soil but from CO2 and H2O.
Not all types of organisms have the same mineral requirements. For instance, while sodium (Na) is an essential element for mammals, it is considered merely beneficial for plants. The same applies to cobalt (Co). Plants do not require fluorine (F); mammals do. On the other hand, boron (B) is essential for plants but not for other organisms. In total, 14 elements (besides C, H and O) have been determined as being essential for plants (Fig. 7.1).
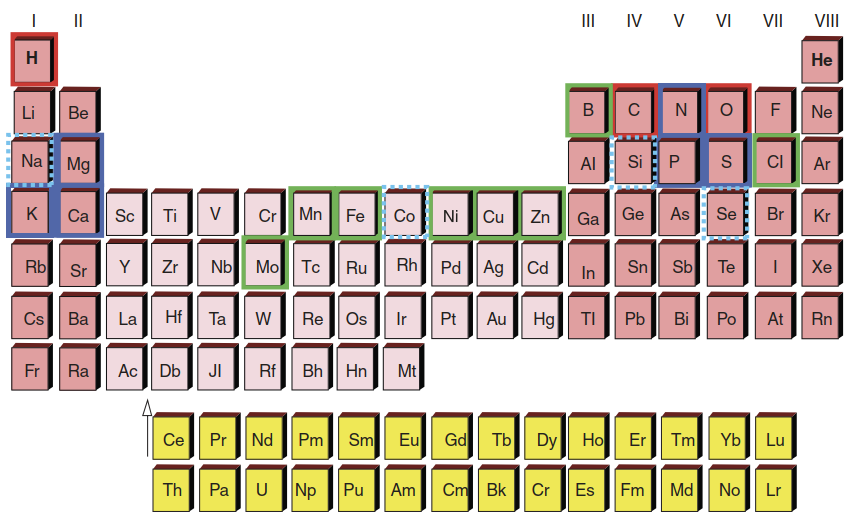
Fig. 7.1. The elements of life from a plant’s perspective: macroelements (blue frame), microelements (green frame) and beneficial elements (dotted line)
It was a fundamental insight that in principle every mineral element can become limiting for plant growth, independently of the amount that is required. What matters is the ratio between the requirement for and the availability of every nutrient. This is described as Justus Liebig’s law of the minimum. In an extreme case an element could become limiting if it is used by only a single indispensable protein but is not available in sufficient amounts to supply this one enzyme. Deficiencies cause symptoms characteristic of the element that is limiting. For example, Mn deficiency causes yellowing of the young leaves in dicotyledonous plants, while the major symptom in cereals is the development of grey specks in the mature leaves (Marschner 2012).
Since practically every natural ecosystem responds to mineral nutrient input, normally by enhanced biomass production, it can be inferred that nutrient limitation is very common or, in other words, that plants are practically always under stress from suboptimal availability of mineral nutrients. Depending on the habitat, different elements tend to become limiting. Alkaline soils are often Fe limited because of very low Fe availability. However, the predominant limitations for plants are nitrogen and phosphate deficiency. The use of N and P fertilisers is one major reason for the enormous yield increases seen in many regions of the world over the past 100 years.
The functions of essential and beneficial mineral elements in plants are very diverse. They are usually categorised into four groups based on their physiological context and biochemical properties (Taiz and Zeiger 2006). Group 1 contains nitrogen and sulphur as the elements that are incorporated (assimilated) into carbon compounds. N is the most abundant of the mineral elements, since it is part of proteins and nucleic acids. S is part of two amino acids and of several essential metabolites such as coenzyme A or glutathione (GSH). Group 2 elements are P, B, and Si, which are important for energy storage or structural integrity. P is a component of nucleotides, nucleic acids and phospholipids.
Myriad small molecules and macromolecules in cells can become phosphorylated. Boron is complexed by several components of the cell wall. Group 3 comprises the elements that remain in ionic form inside plants: the macroelements K, Ca and Mg; the microelements Mn and Cl; and the beneficial element Na. K+ ions are the major osmoticum in plant cells at concentrations around 100 mM. Ca2+ ions are components of the cell wall and inside cells are involved in a vast number of signal transduction processes as second messengers (see, for instance, the common sym pathway, discussed in Sect. 7.4.3).
Finally, group 4 encompasses microelements involved in redox reactions: Fe, Cu, Zn, Ni and Mo. Fe and Cu are redox-active metals, which can exist in two different oxidation states under physiological conditions. This is the reason why they have been recruited for electron transfer reactions in biological systems, most prominently seen in photosynthesis and respiration (e.g. Fe-S proteins, plastocyanin, cytochrome c). Zn is a widely used cofactor in enzymes from all six enzyme classes. Mo plays a key role in the global nitrogen cycle as a cofactor of nitrate reductases and nitrogenases. The predominant use of microelements in enzymes explains why they are required in smaller amounts than the main osmotica (K) or the components of all major macromolecules (N).
Date added: 2025-01-27; views: 352;
