Responses to Element Toxicity and Tolerance Mechanisms
Practically all plants exhibit some degree of basal tolerance of element toxicity. This can be easily demonstrated by studying mutants lacking a gene contributing to this basal tolerance. They are hypersensitive because of the gene loss (Fig. 7.25).
Responses to toxic concentrations of elements, as well as basal cellular tolerance mechanisms, are described in this chapter. Also included are molecular detoxification pathways underlying within-species differences in tolerance, and adaptations found in plants thriving in habitats characterised by extremely unfavourable soil chemistry. These specialists’ tolerance is sometimes referred to as naturally selected hypertolerance. In this case, “tolerance” and “hypertolerance” are the preferred terms, rather than “resistance”. The latter can be misunderstood as implying that no negative effects occur regardless of the concentrations (this, for instance, applies to antibiotic resistance of bacteria or disease resistance of plants and animals). In contrast, the ability to withstand element toxicity is a gradual phenomenon. At a certain concentration, even the most tolerant organisms will suffer from growth inhibition.
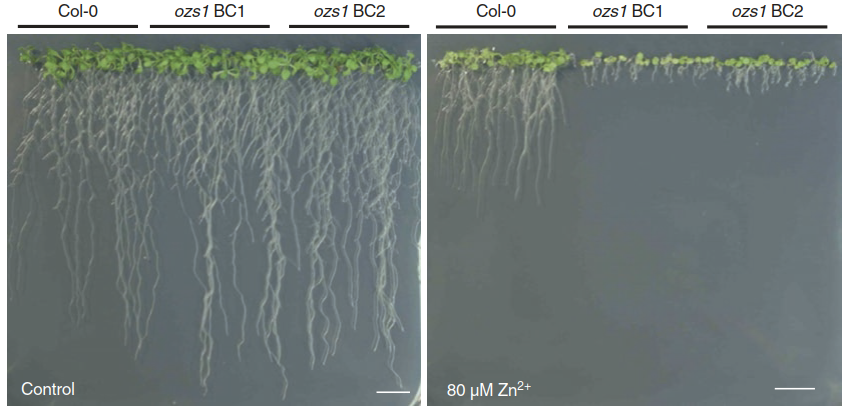
Fig. 7.25. Loss of a basal metal tolerance mechanism results in metal hypersensitivity. A typical metal tolerance assay with Arabidopsis thaliana seedlings is shown. In the presence of an excess of Zn2+ ions, growth of the mutant ozsl is more strongly inhibited than that of the wild type (Col-0). The ozsl mutant carries a defect in the transporter MTP1, which mediates vacuolar sequestration of Zn. BC backcross. bar = 1 cm (Weber et al. 2013)
Essential Metal Toxicity and Tolerance. The essential element boron has a particular narrow optimal range (Fig. 7.2a). Like boron deficiency, boron toxicity limits agricultural productivity in many regions around the world. B toxicity occurs frequently in arid environments on soils rich in B, such as alkaline soils of marine origin. It was first described in southern Australia. Typical symptoms are leaf burns—necrotic spots at the tips of older leaves where higher concentrations of B accumulate because of the transpiration stream that moves mineral nutrients with the water (Nable et al. 1997). Tolerance is conferred by transporters mediating the efflux of boron into the root apoplast (Fig. 7.26). This limits the translocation of boron to the shoot. At high external boron concentrations the efflux transporters can lower cytosolic concentrations (Miwa et al. 2007).
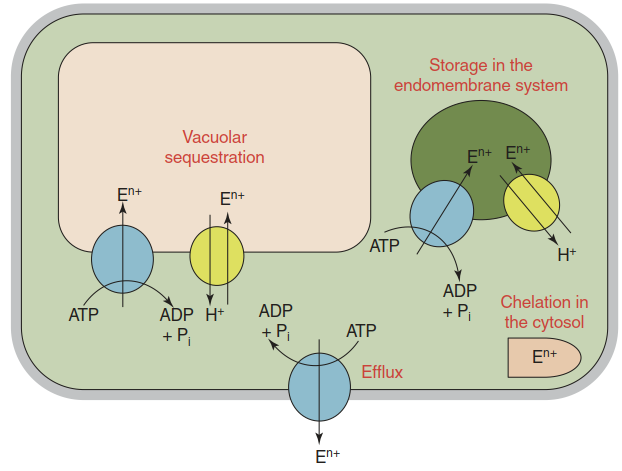
Fig. 7.26. Principal strategies to cope with an excess of a nutrient or a non-essential toxic element. The key is limiting the concentration of a reactive element ion (En+) in the cytosol by sequestration in the vacuole or vesicles of the endomembrane system (e.g. the endoplasmic reticulum (ER) and Golgi), efflux into the apoplast or chelation in the cytosol
There is a documented adaptation to boron toxicity within several crop species, including wheat and barley. Wheat cultivars bred for southern Australia have a yield advantage in their region of origin and perform less well than cultivars from northern Australia when grown there. Genetic loci controlling boron tolerance are known from wheat and barley. Intraspecific differences have been traced to variations in boron efflux transporters. In a highly boron-tolerant barley cultivar this trait is associated with much stronger root expression (> 100-fold higher mRNA abundance) of a boron efflux transporter, which is at least partly attributable to an increase in the gene copy number (Sutton et al. 2007).
After Fe, Mn is the second most abundant transition metal in the Earth’s crust. Mn availability is positively correlated with proton concentrations in the soil solution; thus, in acidic soils Mn can become toxic to plants. A second factor causing increasing Mn availability up to potentially growth-inhibitory concentrations is the reducing condition of waterlogged soils, which promotes the more bioavailable Mn(II) over the much less available Mn(IV) (Foy et al. 1978). Consequently, plant species adapted to growth under such conditions can tolerate much higher Mn content in their tissues. Rice, for example, can cope with a Mn content of 5000 qg/g d.w. in leaves, while many other plants show toxicity symptoms when the Mn content in the leaves exceeds 150 qg/g d.w. (Marschner 2012).
The dominant metal tolerance mechanism is compartmentation. An excess of metal is removed from the cytosol to prevent deleterious effects. In the case of Mn, transporters in the MTP family (metal tolerance proteins; the original name is CDF, for cation diffusion facilitator) mediate sequestration of Mn in the vacuole as the major storage site or in the endoplasmic reticulum (ER) and Golgi (Fig. 7.26).
The transporters are present in plants with basal tolerance, as well as those with specific adaptations. Rice is highly Mn tolerant and MTP proteins are involved in Mn tolerance. The same applies to Stylosanthes species (e.g. S. hamata)—tropical legumes thriving in acid soils. However, to date it is not clear whether differences in expression or specific properties of these transporters in the Mn-tolerant species explain adaptation to high-manganese soils. Alternative compartmentations away from the cytosol are accumulation in the apoplast and exclusion from plant tissues. A possible mechanism, similar to the mode of Al tolerance, is the secretion of organic acids that form 1:1 complexes with Mn.
Date added: 2025-01-27; views: 307;
